Thermal Expansion of TiS: Assessment of Miscibility with Troilite (FeS)
R. Skála1, M. Drábek2, T. Boffa-Ballaran3
1Institute of Geology, ASCR, Rozvojová 269, 165 00 Praha 6
2Czech Geological Survey, Geologická 6, 152 00 Praha 5
3Bayerisches Geoinstitut, Universität
Bayreuth, D-95440 Bayreuth, Germany
skala@gli.cas.cz
From meteorites - aubrites, in which
otherwise lithophile elements behave as siderophiles due to strongly reducing
conditions, titanium-rich iron monosulfides were reported in literature. For
example, in the Bustee aubrite, the titanium-bearing troilite, associated with
osbornite (TiN), heideite (FeTi2S4) and oldhamite (CaS),
was found to contain 17.2 to 25.2 wt % Ti. In the Bishopville aubrite, the
content of titanium in troilite is reported to be up to 5.7 wt % [1]. The
crystal structures of troilite and TiS are not identical under ambient
conditions. While troilite is hexagonal with space group is P![]() 2c and unit-cell dimensions a ~ 5.97 Å, c ~
11.76 Å, V ~ 362 Å3 [2], TiS adopts NiAs-type structure
with space group P63/mmc and the unit cell parameters a
~ 3.31 Å, c ~ 6.34 Å, V ~ 60.2 Å3
[3]. At elevated temperatures, however, the troilite structure transforms to
NiAs-type structure as well [4]. Consequently, a relatively significant mutual
solubility can be expected between FeS and TiS at temperatures above this phase
transition. To evaluate the crystallographic limits for the TiS miscibility in
FeS we carried out a series of high-temperature unit-cell refinements for the former
phase.
2c and unit-cell dimensions a ~ 5.97 Å, c ~
11.76 Å, V ~ 362 Å3 [2], TiS adopts NiAs-type structure
with space group P63/mmc and the unit cell parameters a
~ 3.31 Å, c ~ 6.34 Å, V ~ 60.2 Å3
[3]. At elevated temperatures, however, the troilite structure transforms to
NiAs-type structure as well [4]. Consequently, a relatively significant mutual
solubility can be expected between FeS and TiS at temperatures above this phase
transition. To evaluate the crystallographic limits for the TiS miscibility in
FeS we carried out a series of high-temperature unit-cell refinements for the former
phase.
To collect the diffraction data we used an X’Pert PRO MPD Alpha-1 multi-purpose X-ray diffraction system equipped with an incident beam monochromator, Co tube, and X’Celerator detector. The material was a synthetic TiS prepared under controlled condition in an evacuated silica tube. The sample was heated in an HTK 1200 high-temperature chamber in an alumina sample holder. The holder spun. The NIST silicon internal standard was used for calibration. To prevent oxidation of the sample helium protecting atmosphere was utilized.
In Figure 1 we present results of high-temperature diffraction study of TiS for temperature range from 20 to 400 °C. Within the interval the parameter a and the cell volume increase monotonically whereas cell edge c has the opposite trend. This behavior is identical to that observed for (close-)stoichiometric FeS above the temperature of transition between 2H and 1C polytypes [4]. This observation substantiates a broad field of mutual solubility along FeS - TiS join at elevated temperatures. On the contrary, these results do not corroborate that phases described from aubrites posses the troilite type 2H structure; most probably the transformation which troilite undergoes at ca 100 °C causes the change in symmetry in Ti-containing minerals.
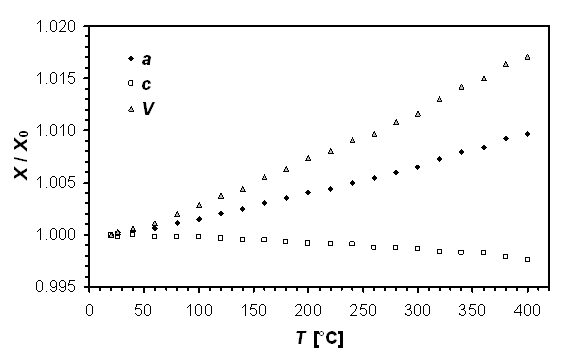
Figure 1. Relative changes of unit-cell dimensions
in TiS at elevated temperature.
1. D.W. Mittlefehldt, T.J. McCoy, C.A.Goodrich, A. Kracher, in Planetary Materials, edited by J.J. Papike (Washington: Mineralogical Society of America), 1998, 195.
2. H.T. Evans
Science, 167, (1970), 621.
3. H. Hahn & B. Harde Zeit. Anorg.
Allgem. Chemie 288, (1956), 241.
4. E.N. Selivanov, A.D. Vershinin, R.I. Gulyaeva Inorg. Materials, 39, (2003),1097.