SYNTHESIS AND STRUCTURE OF CoGeTe AND CoSn1.5Te1.5
F. Laufek1, J. Navrátil2, M. Plášil3
1Czech Geological Survey, Geologická 6, Praha 5,
152 00, Czech Republic
2Joint Laboratory of Solid State Chemistry of IMC AS ČR and University of Pardubice, Studentská 84, 532 10 Pardubice, Czech Republic
3Charles University, Faculty of Science, Albertov 6, Praha 2, 128 43, Czech Republic
laufek@cgu.cz
This presentation is a part of systematic
investigations on crystal structures and physical properties of M-X-Ch compounds
of cobalt-group metals (M=Co, Ir, Rh) and main group IV and VI elements (X =
Si, Ge, Sn; Ch = S, Se, Te). These phases are of
interest in materials science because of their possible thermoelectric
applications.
The ternary compounds, CoGeTe and CoSn1.5Te1.5 were synthesised from the elements by high temperature solid state reactions. Co powder was at first heated at 800 °C for 1 hr in H2 atmosphere to remove possible oxides. Stoichiometric amounts of Co (99.99%), Ge (99.99%), Sn (99.99 %) and Te (99.99%) were sealed in evacuated quartz tubes and heated at 1150 °C for 4 hrs. Following this, the samples were ground using the agate mortar and pestle, and sealed again under vacuum in quartz tubes. The mixtures were heated at 550 ˚C for three days. The resultant material was once again ground and heated at 670 °C and 600 ˚C for CoGeTe and CoSn1.5Te1.5, respectively. After long-term annealing, the samples were quenched in cold water.
Here we report a detailed structural study on the two ternary compounds CoGeTe and CoSn1.5Te1.5. As single crystals of sufficient quality were not available, the analyses were performed on powder samples. The crystal structure of CoGeTe was solved by direct methods by means of EXPO2004 [1] program package, while the inicial structure model for CoSn1.5Te1.5 was derived from data published for CoGe1.5Te1.5 [2]. Both structures were refined by Rietveld method by means of FullProf program [3].
CoGeTe:
space group Pbca, a =
6.1892(4), b = 6.2285(4), c = 11.1240(6) Å, Z = 8, Rwp
= 0.083, RB = 0.065. The crystal structure of CoGeTe can be
viewed as a ternary ordered variant of α-NiAs2 (also known as a
mineral pararammelsbergite), which is transitional between the marcasite-type
and the pyrite-type structures. Each Co atom is surrounded by three Ge and Te
atoms showing a distorted octahedral coordination. One octahedral edge is
shared with an adjacent octahedron, compared to two shared edges in the
marcasite structure and none in the pyrite structure. Other vertices of the
[CoGe3Te3] octahedron are connected by corners sharing.
(Fig. 1). Similar description has been described for PtSiSb [4].
CoSn1.5Te1.5: space group R-3, a = 12.9062(2), c = 15.7837(3)
Å, Z = 12, Rwp = 0.106, RB =
0.046. The structure of
CoSn1.5Te1.5 can be described as a modification of a
cubic structure of CoSb3 [5] (skutterudite type). The weak
superstructure reflections found in powder diffraction pattern reveal the
ordering between Sn and Te atoms. This ordering reduces the symmetry from cubic
to rhombohedral. Each Co atom is surrounded by three Te and Sn atoms forming a distorted octahedral coordination. The [CoSn3Te3]
octahedra share only corners with six neighbouring octahedra. One of the most
characteristic features of the CoSn1.5Te1.5 structure is
the presence of two distinct four-member rings [Sn2Te2]
(Fig 2). The schematic representation of the CoSn1.5Te1.5
structure is shown in the Fig. 2. A compound with this composition was
mentioned at the conferences [6, 7] but no structural details were given.
1. A. Altomare, R. Caliandro, M. Camalli, C. Cuocci, C. Giacovazzo, A. Moliterni. R. Rizzi, J. Appl. Cryst., 37, (2004), 1025.
2. P. Vaqueiro, G.S. Sobany, A.V. Powell, K.S. Knight, J. Solid State Chem., 179, (2006), 2055.
3.
J. Rodríguez-Carvajal, FullProf.2k, Laboratoire Léon Brillouin, France,
2006.
4. M. Wang, M.G. Morgan, A. Mar, J. Solid State Chem., 175, (2003), 231.
5. Th. Schmidt, G. Kliche, H. D. Lutz, Acta Crystallogr.. C 43, (1987), 1978.
6. Y. Nagamoto, K. Tanaka, T. Koyanagi, Proceeddings of the 16th International Conference on
Thermoelectrics, Dresden, Germany.
7.
A. Smaleez, Q. Lin, D. C. Johnson, J. Mertin, Material Research Meeting
2005, p. 137., Boston, USA..
This study was supported by the Grant Agency of the Academy of Sciences
of the Czech Republic (Project No. KJB 300130612), by the internal project of
the Czech Geological Survey (Project No. 3230) and by the Czech Science
Foundation (Project No. 203/07/0267).
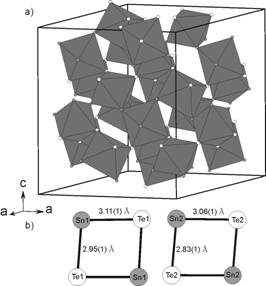
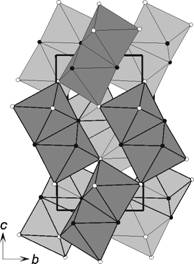
Figure 1. Polyhedral representation of CoGeTe Figure 2. (a) Polyhedral
representation of CoSn1.5Te1.5
structure showing the [CoGe3Te3]
octahedra. Black structure
showing the corner sharing arrangement of
and white circles are Ge and Te atoms,
respectively. the [CoSn3Te3] octahedra. (b) Four-member Sn2Te2
rings.