Solid-state synthesis, characterization and applications of potassium ferrate(VI): A multi-analytical approach
J. Filip1, L. Machala1, R. Zboøil1, V. K. Sharma2, I. Medøík1
1Centre for nanomaterial research, Palacký University Olomouc, Svobody 26, 771 46 Olomouc, Czech Republic
2Chemistry department, Florida Institute of Technology, 150 West University Boulevard, Melbourne, FL 32901, USA
jan.filip@upol.cz
In the recent years, there has been increasing interest in the +6 oxidation state of iron. The ferrate(VI) ion (FeVIO42-) is the only one known species [1], important because of its potential use in high energy density rechargeable batteries, in “greener” technology for organic synthesis, and in treatment of contaminants and toxins in water and wastewater [2]. Potassium ferrate(VI) (K2FeO4) is the most studied compound among the family of ferrate(VI) [2]. Hence, solid orthorhombic, few micrometer-sized crystals of potassium ferrate(VI) were synthesized by thermally induced solid-state reaction, where KNO3 and suitable iron-containing compounds (mostly cheap waste iron oxides, oxyhydroxides and sulfates, available in high quantities) were heated together to obtain K2FeO4. However, the immediate decomposition of ferrate(VI) occurs at elevated temperatures used in a thermal synthesis technique [3], which results in a low yield of K2FeO4 product. An increase in the K2FeO4 yield was achieved by optimizing the precursor composition and the temperature conditions under which the secondary decomposition is reduced. 57Fe Mössbauer spectroscopy and X-ray powder diffraction analyses were routinely used to monitor the process of synthesis.
Resulting potassium ferrate(VI) (space group Pnam) reveals the refined lattice parameters, a = 7.702(2), b = 10.346(1), c = 5.862(1) Å, and cell volume 467.1(1) Å3, which are close to those reported by other researchers. Potassium ferrate(VI), or mixture of potassium ferrate(VI) and potassium iron(III) oxide (KFeO2) (Fig. 1a,b), depending on the procedure used, was found to be very effective in technology of water treatment under laboratory conditions [2, 4]. In all cases, the final reaction product of potassium ferrate(VI) decomposition and Fe6+–to–Fe3+ reduction is two- to six-line ferrihydrite (Fe5HO8·4H2O) (Fig. 1c,d) of large surface area and nanocrystalline character (the mean particle size of ferrihydrite calculated from broadening of diffraction peaks according to the Scherrer formula and confirmed by transmission electron microscope equals to mean 5 nm), or mixture of ferrihydrite with better crystalline phases (e.g., goethite), depending on the type of pollutants and reaction kinetics. Just the nanocrystalline character and large surface area (up to 183 m2/g measured using a Brunauer-Emmett-Teller – BET – surface area analyzer) of final iron oxyhydroxides is a unique phenomenon, great for a possible usage of potassium ferrate(VI) in water treatment technologies. Hence, the initially oxidized toxic metals and other pollutants are effectively adsorbed on and/or co-precipitated with the subsequently formed iron oxyhydroxides.
57Fe Mössbauer spectroscopy (room- and low-temperature, and in-field configuration), together with X-ray powder diffraction, represent powerful tools in monitoring the phase composition and structural properties of the synthesized potassium iron(III, VI) oxides, their stability and reaction mechanisms with pollutants. Mössbauer spectroscopy gives a unique information about the oxidation state of iron, phase composition and phase ratio of iron-bearing and even amorphous phases, whereas X-ray powder diffraction brings the possibility to add both qualitative and quantitative data to all presented, mainly crystalline phases. The presented results have a practical impact for the optimization of the solid-state synthesis of potassium ferrate(VI). Moreover, new data will be presented in terms of phase composition of reaction products of potassium ferrate(VI), as well as potassium iron(III) oxide and their mutual mixture.
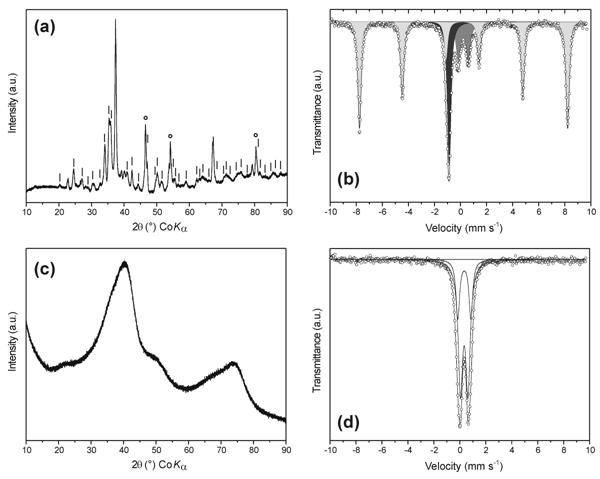
Figure 1. (a) X-ray powder diffraction pattern of synthesized mixture of K2FeO4 and KFeO2, vertical lines indicate X-ray diffraction peaks of K2FeO4, circles mark the Pt-holder as the pattern was measured under vacuum to protect the sample reaction with air moisture during the experiment; (b) 57Fe Mössbauer spectrum of synthesized mixture of K2FeO4 and KFeO2, dark gray – subspectrum corresponding to K2FeO4, light gray - KFeO2, medium gray – amorphous iron oxyhydroxides; (c) X-ray powder diffraction pattern and (d) 57Fe Mössbauer spectrum of two-line ferrihydrite forming during the reaction of K2FeO4 with pollutants in aqueous solution.
1. J. F. Berry, E. Bill, E. Bothe, S. D. George, B. Mienert, F. Neese, K. Wieghardt, Science, 312, (2006), 1937-1941.
2. V. K. Sharma, Adv. Environ. Res, 6,
(2002), 143-156.
3. L. Machala, R. Zboøil, V. K. Sharma, J. Filip, O. Schneeweiss, Z. Homonnay, J. Phys. Chem B., 111, (2007), 4280-4286.
4. V. K. Sharma, F. Kazama, H. Jiangyong, A. K. Ray, J. Water Health, 3, (2005), 45-59.
Financial supports from the Ministry of
Education of the Czech Republic (MSM6198959218 and 1M6198959201) and from
Academy of Sciences of the Czech Republic (KAN108040651) are gratefully
acknowledged.