Neutron diffraction, INS and DFT study of hydrogen-bonding in TRIS
Mariana Sládkovičová1, Pavel
Mach1, Ľubomir Smrčok1, Paula Marie Briggs-Piccoli2, Alexander
Kolesnikov2
1Institute
of Inorganic Chemistry, Slovak Academy of Sciences, SK-845 36
2Argonne
National Laboratory,
 The proposed project is a part of the study focusing on detailed
description of H-bonds in the selected compounds by making a joint use of
diffraction, spectroscopy and quantum chemistry methods. The compound
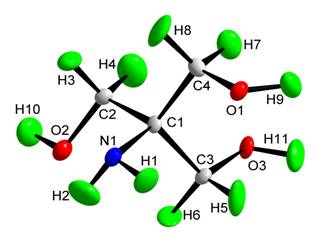
tris(hydroxymethyl)-aminomethane, H2NC(CH2OH)3,
commonly known as TRIS (see the Figure below) can be considered as a good model
system for studying the electronic structure of hydrogen bonded-solids. The
structure of TRIS can be considered as a layer structure with both intra- and
interlayer hydrogen bonds. The individual layers are, except for long range
electrostatic forces (dipole moment of the molecule is 2.15D), also connected
by weak interlayer hydrogen bonds linking N-H…O. Such a structure
provides a challenge for theoretical approaches to hydrogen bonded systems
since hydrogen bonding can to great extent influence the geometry of a hydrogen
bonded molecule and its dynamics.
The proposed project is a part of the study focusing on detailed
description of H-bonds in the selected compounds by making a joint use of
diffraction, spectroscopy and quantum chemistry methods. The compound
tris(hydroxymethyl)-aminomethane, H2NC(CH2OH)3,
commonly known as TRIS (see the Figure below) can be considered as a good model
system for studying the electronic structure of hydrogen bonded-solids. The
structure of TRIS can be considered as a layer structure with both intra- and
interlayer hydrogen bonds. The individual layers are, except for long range
electrostatic forces (dipole moment of the molecule is 2.15D), also connected
by weak interlayer hydrogen bonds linking N-H…O. Such a structure
provides a challenge for theoretical approaches to hydrogen bonded systems
since hydrogen bonding can to great extent influence the geometry of a hydrogen
bonded molecule and its dynamics.
Accurate structure of TRIS was obtained from low temperature single crystal neutron diffraction. In the molecule of TRIS there are three -OH groups and one - NH2 group that could act both as donors and acceptors of hydrogen bonds. The INS spectrum of TRIS was measured at 7 K on the HRMECS spectrometer with several incident neutron energies and was interpreted on the basis of a harmonic potential using a variety of models. The models were a single molecule, a molecule in a static field of fragments, and single unit cell with periodic boundary conditions as implemented in VASP program. INS spectrum of TRIS is basically a spectrum of hydrogen motions. Formation of total of six inter-molecular hydrogen bonds caused significant downshift of OH stretching frequencies and upshift of their bending frequencies. The same trend was observed for NH2 frequencies but the changes were smaller. This finding goes in agreement with refined structure, which showed that NH2 participates in the longest hydrogen bond. Hydrogen bonding has almost no influence on CH frequencies. When comparing calculated INS spectra for all models with experimental spectrum the best agreement was reached when considering not only closest hydrogen bonded neighbors but also the periodic character of the crystal structure.