One Example of Useful Disorder : Structure of Pr(III) Complex of 1,4,7,10-Tetraazacyclododecane-10-methyl-1,4,7-tris(methylenephenylphosphinic) Acid
Jana Klimentová, Pavel Vojtíšek *
Department of Inorganic Chemistry, Faculty of Science, Charles University, Hlavova 2030, Prague 2, 128 43, Czech Republic, * pavojt@natur.cuni.cz
Coordination chemistry of lanthanide ions and yttrium with N,O-macrocyclic ligands is widely investigated because of the importance of their medicinal and biochemical use, such as Gd3+ complexes as contrast agents (CA) in magnetic resonance imaging (MRI) [1,2], 90Y complexes in radioimmunotherapy [2] and luminiscent Eu3+ and Tb3+ compounds as probes [2,3]. Previously, we published [4] an interesting set of structures of complexes of 1,4,7,10-tetraazacyclododecane-10-methyl-1,4,7-tris(methylenephenylphosphinic) acid (H3L, see Figure 1). This acid forms neutral dimeric complexes [LnL(H2O)n]2. x H2O. y MeOH with Ln3+ ions, where n = 0 or 1, x = 5-7 and y = 0-2, for different Ln3+ [4]. All compounds are isostructural and crystallise in space group P 21/c (no. 14) with similar values of lattice parameters and similar position of the lanthanide ion in the unit cell.
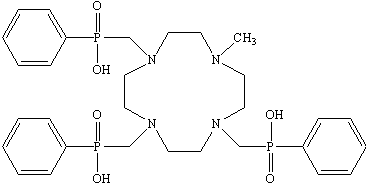
Figure 1: The formula of H3L.
However, the compound [PrL(H2O)n]2. x H2O does not belong to the previously published isostructural set. The structure investigation of [PrL(H2O)n]2. x H2O has resulted in an interesting conclusion [5]. The successful modelling of disorders in this case gave us a picture of “frozen solution” with samples of isomeric species present. Three chemically and four crystallographically different complexes were identified and structurally characterised: [Pr{L(R,S)}]2 with coordination number (CN) 8, [Pr{L(R,R)}(H2O)]2 with CN 9 and two species [Pr{L(R,S)}(H2O)]2 also with CN 9, but with a little different geometrical parameters (the symbols L(R,R) and L(R,S) mean the different chirality on phosphorus atom of the ligand labelled P2 in the resulting complex, respectively). Therefore, the title compound should be written as 0.67 [Pr{L(R,R)}(H2O)]2 . 0.33 [Pr{L(R,S)}(H2O)]2 . 0.67 [Pr{L(R,S)}]2 . 0.33 [Pr{L(R,S)}(H2O)]2 . 27.5 H2O.
In our opinion, it illustrates that disorder can represent not only a nuisance in structure solving and refinement; it may bring useful chemical information as well.
1. The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, eds. A. E. Merbach, É. Tóth, Wiley, Chichester, U. K. (2001); Topics in Current Chemistry, Springer, Frankfurt am Main, Germany 221 (2002).
2. D. Parker, in Comprehensive Supramolecular
Chemistry, ed. J.-M. Lehn,
Pergamon, Oxford, 10 (1996) 487.
3. S. Faulkner, J. L. Matthews, in Comprehensive Coordination Chemistry II, eds. J. A. McCleverty, T. J. Meyer, Elsevier, Amsterdam 9 (2004) 913 and refs. therein.
4. J. Rohovec, P. Vojtíšek, I. Lukeš, P. Hermann, J. Ludvík, J. Chem. Soc., Dalton Trans. (2000) 141.
5. J. Klimentová, P. Vojtíšek, J. Mol. Struct., accepted for publication.