LOD METHOD FOR DETECTION OF LOW AMOUNT OF ONE POLYMORPH IN ANOTHER ONE
Hana Petříčková
Zentiva a.s.
R
& D Laboratories of Solid Phase Analysis, U kabelovny 130, 102 37 Prague – 10, Czech
Republic
Modern
pharmaceutical R&D analytical department is toothless without solid state
characterization techniques. XRPD plays
at this field essential role.
Crystallization
is one of the most important processes during the production of
pharmaceuticals. Polymorphic behaviour of pharmaceutical substances is common
as well as the diffraction pattern of such polymorphs are different.
Control of
polymorphic purity may be crucial from many points of view such as stability,
dissolution or patent infringement. Also LOD (limit of detection) determination
is part of validation method of the identification test specifications.
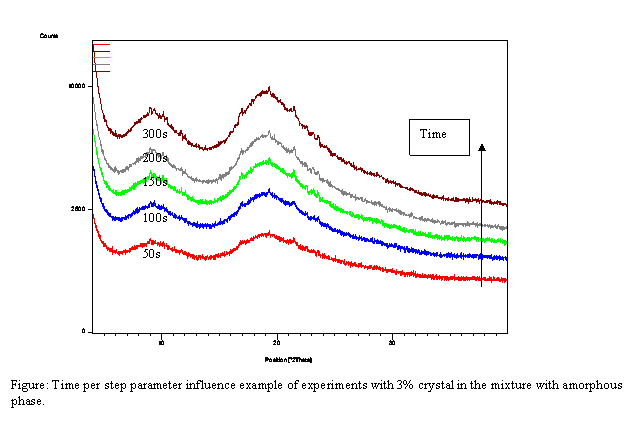
To detect low
amounts of unwanted polymorph, which can be present in large scale produced
polymorph, the powder X-ray diffraction method was developed. Results of two
case studies will be presented. LOD determination of crystalline polymorph II
in polymorph I. and LOD determination of crystalline polymorph I. in amorphous
material will be presented. Produced polymorphic form I is more stable and
better soluble comparing to form II., presence of form II is undesirable. XRPD
is also used as a negative evidence of amorphous form, no sharp peaks are
satisfactory. Corresponding diffractometer
setting and key parameter will also be discussed.