CRYSTALLOGRAPHY
OF THE Sb-Te-Ni SYSTEM
F. Laufek1,3,
M. Drábek1, R. Skála2, I. Císařová3
1Czech
Geological Survey, Geologická 6, Praha 5, 152 00, Czech Republic
2 Institute of Geology, Academy of Sciences of the Czech Republic, Rozvojová 135, Praha 6, 165 02, Czech Republic
3 Faculty of Science, Charles University, Hlavova 8, , Praha 2, 128 43, Czech Republic
The new unnamed
nickel antimonide telluride Ni2SbTe2 was found as 6
μm grain by Vavřín and Frýda [1] at the Kunratice Cu-Ni deposit (North
Bohemia). This ternary phase is in a close association with melonite (NiTe2);
this assembly is included in pyrrhotite (Fe1-xS). In order to
determine the crystal structure of this phase (from the synthetic analogue) and
to further explore the Ni-Sb-Te phase diagram, the crystallography of this
ternary system was investigated. The phases were prepared using the silica
glass tube method. Our experiments were performed in the Experimental laboratory
of Czech Geological Survey. High-purity elements – tellurium (99.999 %),
antimony (99.99 %) and nickel (99.995 %) were used as starting materials.
Before being used for syntheses, nickel was heated for 1 hour in a stream of H2
at 800 ˚C. Carefully weighted samples were loaded into the high purity
silica tubes and tightly fitting silica rods were placed on the top of the
reagents in order to reduce the vapour volume during heating. The silica tubes
with the charge were sealed under vacuum and then heated in the horizontal
furnaces in which the temperature was controlled electronically. The maximum
temperature variation did not exceed 4 ˚C. The samples were heated at
400˚C or at 800˚C for three weeks. The experiments were terminated
either by quenching in a cold bath or by slow controlled cooling to room
temperature.
The crystal
structure of the new phase Ni2SbTe2 prepared at
800˚C (experiment terminated by quenching), determined from X-ray single
diffraction data, is hexagonal, NiAs type, with lattice parameters: a = 3.9108(2), c = 5.2489(3) Å, space group P63/mmc (no.
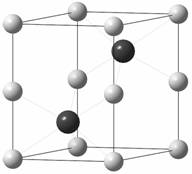
194). The antimony and tellurium occupy the crystallographic position 2c; the position 2a is occupied by nickel atoms (Fig. 1)
The crystal
structure of Ni2SbTe2 prepared at 400 ˚C (experiment
terminated by slow controlled cooling to room temperature within interval 22
hours), originally described by [2], refined from single X-ray diffraction
data, is hexagonal with lattice parameters
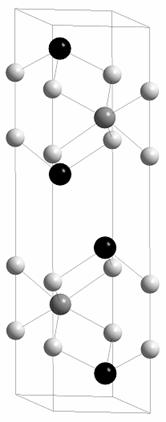
a = 3.9110(2), c = 15.696(1) Å, space group P63/mmc (no. 194). The antimony and tellurium atoms occupy different
crystallographic positions, antimony 2c
and tellurium 4f. (Fig. 2). The
crystal structure can be described as “hybrid” of NiSb and NiTe2
with an elongated c-axis. In NiSb structure, the close packed anion layers are
all Sb with ABAB stacking and all octahedral holes are filled with Ni. If every
third plane of Ni atoms is removed from NiSb and Sb replaced with Te in two out
of three layers, we can obtain the Ni2SbTe2 structure
(low temperature modification). The atom stacking sequence is CABCAC (A – Ni, B
– Sb, C – Te). The interlayer Te – Te distance is 3.543 Å. It is also to
be noted that this interatomic distance is larger than Te2
pair-cointaining phases (2.763 Å in HfTe2 and 2.793 Å in
ZrTe2 ) or in elementary Te (2.84 Å) [3], but shorter than the van der Waals distance
4.12 Å
(default van der Waals radius for Te is taken
from [4]). The weak
interlayer Te bonding results in a layered structure which corresponds to
plate-like morphology of crystals.
The situation
in the case of crystal structure of Ni2SbTe2 prepared at
400˚C (experiment terminated by quenching) is more complicated.The X-ray
powder diffraction pattern corresponds to the high temperature phase,
nevertheless diffraction profiles of 201
and 110 lines are asymetrical. This
assymetry disappears in the powder pattern of Ni2SbTe2
prepared at 800 ˚C (experiment terminated by quenching, Fig. 3). On the
Selected Area Electron Diffraction (SAED) pattern of reciprocal planes h0l it is possible to observe weak
reflections near 1/3 and 2/3 of the distance between the sharp strong
diffractions. These weak reflections are systematically shifted from 1/3 to the
left and from 2/3 to the right, i.e. closer to the sharp strong diffraction.
In fact, there
is a large number of compounds that exhibit Sb – Te bonding in which Sb is
formally cationic. Examples of such compounds include K3SbTe3,
TlSbTe2, SnSb2Te4, BaSbTe3 and NaSbTe2 [2]. In many compounds the Sb and Te atoms are
mixed on the same crystallographic site and thus are not new structure types.
These include e.g. Ni2SbTe, Mn2SbTe, Co2SbTe,
FeSbTe, Pd2SbTe and Cr2SbTe [5]. Virtually, there are
very few compounds which have Sb and Te atoms on distinct crystallographic
positions. Examples of such compounds are Ni7-δSbTe2,
Cu9.1Sb3Te and the new compound Ni2SbTe2.
Our research showed that the crystal structure of this phase depends on the
temperature during the formation.
The phase Ni2SbTe2 forms a solid solution with end members having a composition of 42,1 % Ni, 13,0 % Sb, 44,9 % Te and 43,0 % Ni, 28,4 % Sb, 28,6 % Te (at.%) at 400ºC. The most characteristic feature is a small change of the nickel content as well as significant differences of the antimony and tellurium content
 This work was supported by
a Grant Agency of Charles University (project number 43-203391).
This work was supported by
a Grant Agency of Charles University (project number 43-203391).
![]()
Fig. 1. Crystal structure of Ni2SbTe2 prepared at 800˚C (experiment
terminated by quenching)
![]()
Fig. 2. Crystal structure of Ni2SbTe2 prepared at 400˚C (experiment terminated by slowly cooling to room temperature)
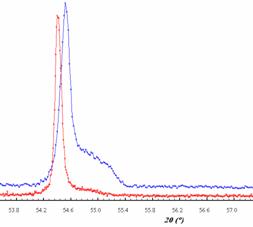
Fig. 3. Diffraction profile of 110 line of Ni2SbTe2 prepared at 400˚C (above) and at 800˚C, CoKα1
1. Vavřín, I. & Frýda, J. Věštník ČGÚ, 73 (1998) 177-180.
2. Reynolds T.K., Kelley R.F. & DiSalvo F.J. J. Alloy Comp., 366 (2004) 136 -144.
3. Bensch, W., Heid. W., Muhler, M., Jobic, S., Brec, R. & Rouxel, J. J. Solid State Chem. 121 (1996) 87 - 94.
4. Bondi, A. J.Phys.Chem. 68 (1964) 441-451.
5. Inorganic Crystal Structure Database (ICSD), 2003