STEREOCHEMISTRY OF CALIX[4]ARENES.
J. Klimentová and P. Vojtíšek
Department of Inorganic Chemistry, Faculty
of Science, Charles University, Prague, Czech Republic
Calix[4]arenes are a fascinating class of
macrocyclic compounds, which has recently attracted a lot of attention because
of their potential wide use in many areas of research and industry. Having
started in the 19th century by reactions of phenol and aldehydes
performed by Adolph von Baeyer; and continued by a considerable effort of David
C. Gutsche in the 1970s, the chemistry of calixarenes has developed into a wide
and well-explored area [1]. Calix[4]arenes have been used principally as
spacers bearing functional groups in a well-defined arrangement, allowing their
desired cooperation [2].
The utilization of calixarenes as molecular
platforms possesses a few advantages. First, the synthesis of these macrocycles
can be easily accomplished by a well-known procedure in good yields. The size
of the macrocycle can be successfully controlled by the reaction conditions
[3]. The starting materials (p-tert.butylphenol and formaldehyde) are
inexpensive and common. Calix[4]arenes can be easily modified both on their
upper and lower rim [3], which allows to change their chemical and physical
properties as required. Finally, the four possible conformations of the
calix[4]arene macrocycle, easily immobilized by lower-rim substitution [2], are
the main reason for the advantage of using calix[4]arenes as molecular
platforms.
Recently, heterocalix[4]arene macrocycles have
been synthesized. These compounds contain a heteroatom (S, N, Si) or a
functional group based on heteroatom (SO, SO2) instead of the
methylene bridge, which is responsible for their greater conformational
flexibility [4].
The conformation and symmetry of the
calix[4]arene molecule is important for its function as a spacer bearing
substituents in a defined arrangement, which allows their interaction,
interaction with cations, anions or neutral molecules, cooperation in ion pair
binding etc. [2, 5]. Another important factor is the rigidity or flexibility of
the substituents and of the calix[4]arene skeleton. The rigidity of the latter
can be achieved by bridging the upper or lower rim of the calix[4]arene
molecule, effectively locking its movements [2]. Furthermore, the conformation
of the calix[4]arene platform can be influenced by the interactions of its
hydrophobic cavity or aromatic rings with cations or neutral molecules by the
means of cation-p interactions, p-p interactions or van der Waals interactions. The substituents on the
upper or lower rim may also participate in shaping of the calix[4]arene
molecule. The possible interactions (beside the above mentioned ones) may
involve inter- or intramolecular hydrogen bonding, electrostatic interactions,
donor-acceptor interactions (cation complexes or Lewis acid-base pairing) and
sterical hindrance. In conclusion, the final shape of the calix[4]arene
platform results from the combination of all these effects.
To elucidate the influence of the substitution on the upper and lower rim of the calix[4]arene and inter- or intramolecular interactions on the conformation of the calix[4]arene molecule, we decided for the Cambridge Structural Database [6] as the largest source of information (about 1,500 calix[4]arene structures). The conformation of the calix[4]arene molecules and inter- or intramolecular interactions of these compounds can be easily determined from the crystal structure data. Nevertheless, this information might not fully correspond to the conformational behavior of the calix[4]arene molecules in solution.
To describe the conformation of the calix[4]arene skeleton, a variety of geometrical parameters can be calculated (e.g. the distances between the oxygen or carbon atoms on the lower or upper rim, the angles of the planes of the phenyl rings etc.). We have decided to describe the calix[4]arene conformation by the defining of a reference plane to which the angles of the four phenyl rings are related. The most convenient reference plane appears to be the plane of the four methylene bridging groups (for the vast majority of structures, the deviation of the methylene carbon atoms from this plane is below 0.01 nm). The angles of the phenyl rings (ai, i = 1-4) are calculated in the scale 0-360º (see Fig. I).
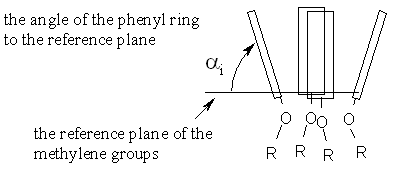
Fig. I : The definition of the phenyl ring angles ai.
Next step in the description of the
calix[4]arene conformations is the definition of geometrical parameters a, b, d according to (1).
a
= 0.25*(a1 +
a2 +
a3 +
a4)
b
= | a1 +
a3 |
- | a2 +
a4 |
(1)
d
= | a1 -
a3 |
+ | a2 -
a4 |
The parameter a is the average value of the phenyl ring angles a1 - a4 (numbering reflects the order of the phenyl rings in the calix[4]arene molecule, e.g. a1, a2 corresponds to adjacent rings, a1, a3 to opposite rings etc.). The parameter b reflects the distortion of the calix[4]arene molecule towards C2v symmetry (for calix[4]arenes in the cone conformation). Finally, d reflects the distortion towards Cs symmetry (again, for calix[4]arenes in the cone conformation). Further examples of the dependence of the parameters a, b, d on the calix[4]arene conformation are depicted in Fig. II (the schemes show slices through the calix[4]arene opposite rings and usual angles).
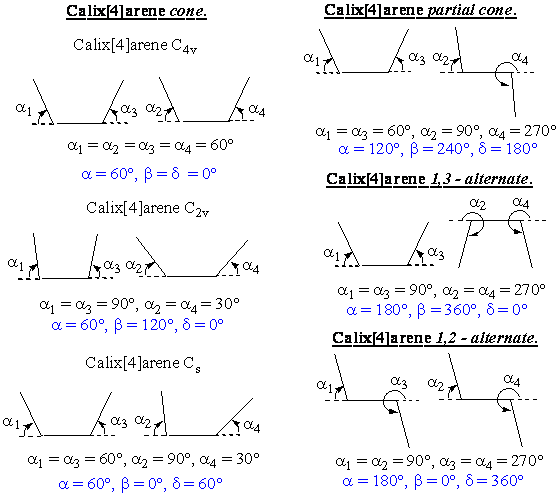
Fig.
II : Parameters a, b, d in
dependence on the calix[4]arene conformation and symmetry.
The parameters a, b, d reflect the conformation of the calix[4]arene molecules (see Fig. II). For example, all calix[4]arenes in the cone conformation have a < 90º and the values of b, d reflect their distortion towards C2v, Cs or C1 symmetry (the latter for both b, d significantly different from zero). The dependence of the b, d values is shown on the group of heterocalix[4]arenes in Fig. III.
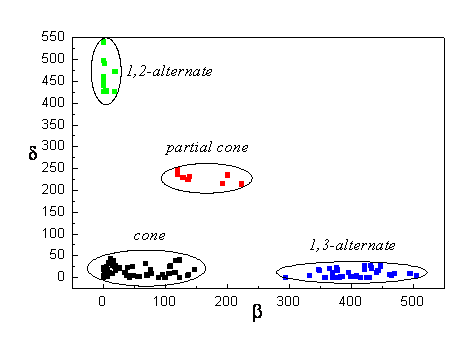
Fig.III
: The distribution of the b, d values in the group of heterocalix[4]arenes from [6].
The dependence of the parameters b, d on the symmetry of the
calix[4]arene is shown on the example of non-complexed calix[4]arenes in the cone conformation symmetrically
tetrasubstituted on the upper and lower rim (Fig. IV).
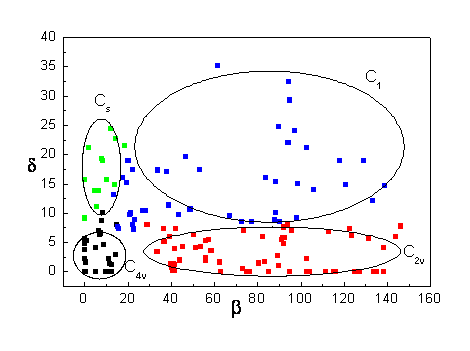
Fig. IV : The dependence of the parameters b, d on the symmetry of the symmetrically tetrasubstituted cone-calix[4]arenes not bound to metal from [6].
The deformation of the symmetrically
tetrasubstituted cone-calix[4]arene
molecules towards C2v, Cs or C1 symmetry is
caused by the above-mentioned types of interactions, principally cation
complexation, p-p stacking, hydrogen bonding and sterical hindrance. Some examples
are given on Fig. V.
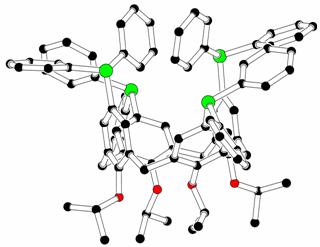
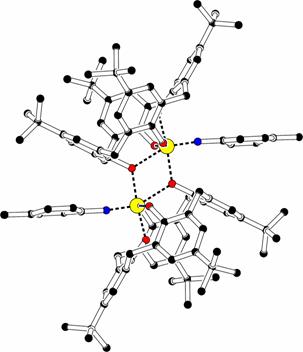
Fig.
V : Examples of deformation of the calix[4]arene molecule towards C2v
and Cs symmetry (atom colors : black C, red O, blue N, green P,
yellow W) [6].
The dependence of the calix[4]arene symmetry on
changing the substitution pattern of the upper or lower rim can be also
considered. Nevertheless, the dependence is complex and results from the
combination of sterical and electronic effects. Our further attempts on this
field are in progress.
Due to the large amount of CSD data and limited
space in this abstract, only a few examples of the influence of the
interactions on the shape of the calix[4]arene molecule are presented.
[1] C.D. Gutsche, Calixarenes, Monographs
in Supramolecular Chemistry, The Royal Society of Chemistry, J.F. Stoddart
, Cambridge 1989
[2] S. Shinkai, A. Ikeda, Chem. Rev., 97 (1997), 1713-1734
[3] Macrocycle Synthesis, editor D.
Parker, Oxford University Press, New York 1996
[4] P. Lhoták, Eur. J. Org. Chem., (2004), 1675-1692
[5] P.D. Beer,
P. Gale, Angew. Chem. Int. Ed., 40 (2001), 486-516
[6] CSD, Cambridge Crystallographic Data
Centre (CCDC)