TANTALONIOBATES IN CASSITERITE: INCLUSIONS OR EXSOLUTIONS?
M. Klementová 1,2, M. Rieder 3
1 Institute
of Inorganic Chemistry,
2 IGMMR,
Faculty of Science,
3
INTRODUCTION
In natural samples, it is sometimes difficult
to differentiate between inclusions that were trapped during crystal growth and
products of post-crystallization exsolution processes. In order to understand
the process of rock formation, the inclusions/exsolutions have to be studied in
detail. Presently, we studied cassiterite from the Annie-Claim Pegmatite (
The word "inclusion" is being used loosely. In general, an inclusion can be any solid or fluid phase enclosed in the surrounding matrix. This covers inclusions sensu stricto, which are randomly trapped by a crystal during its growth, as well as precipitates that form by exsolution in the subsolidus. Hereafter, we shall use the term "inclusion" in non-genetic context, whereas the term "inclusion sensu stricto" as opposed to terms "precipitate" or "exsolution" will allude to the formation process involved.
There are several ways how to differentiate
between inclusions sensu stricto and
precipitates. Under the microscope, a first impression comes from the shape and
spatial distribution of inclusions within the matrix. Inclusions sensu stricto usually are irregular in shape
and are distributed randomly, whereas precipitates form lamellae or assume
other distinct shapes and tend to be arranged in preferred directions, as
demanded by their structural orientation within the host. In the matrix
surrounding a precipitate, a concentration gradient may betray that a diffusion
of elements toward the precipitate took place during its growth; contrariwise,
the matrix around an inclusion sensu
stricto ought to be homogeneous. To obtain yet another piece of evidence,
one might want to perform a single-crystal diffraction experiment in order to
see whether a precipitate is crystallographically aligned in the host. The
underlying idea is that there are hardly any compelling reasons to expect
inclusions sensu stricto to assume a
fixed crystallographic orientation with respect to the matrix.
Tantaloniobates such as ixiolite and columbite have commonly been described as inclusions or precipitates (exsolution products) in cassiterite and rutile [1,2,3,4,5,6,7,8,9,10,11]. However, these studies relied on reflected-light microscopy, microprobe analyses, and X-ray powder diffraction, which may not suffice to differentiate unequivocally between inclusions sensu stricto and exsolution products.
CHEMICAL COMPOSITION
Texture and chemical composition were studied with
the help of electron microscopy (SEM and microprobe). Selected samples were
analyzed quantitatively on the microprobe Cameca SX50 with a Wave Dispersive Spectrometer (operated at 20kV, 25nA) at the
NHM in
In the material studied, it is possible to discern two groups of samples, in agreement with their locality as well as their chemical composition. Canadian samples contain precipitates of monoclinic wodginite MnSnTa2O8, whereas samples from the Czech Massif contain orthorhombic ferrocolumbite Fe(Nb,Ta)2O6. In the samples from both groups, a concentration gradient surrounding the inclusions can be observed (Fig. 1).

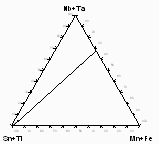

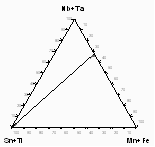
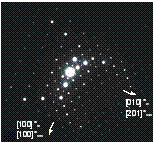
Figure 1: a) Sample from Annie Claim
b) Microprobe analyses of sample 206-IVA from Annie Claim 3 plotted
in triangular diagram Sn+Ti -Mn+Fe - Nb+Ta (at.%).
c) Sample from Nová Ves in back-scattered electrons (BSE). White -
columbite inclusions, light grey - depleted cassiterite, enriched grey -
depleted cassiterite.
d) Microprobe analyses of sample
MM-116 from Nová Ves plotted in
triangular diagram Sn+Ti -Mn+Fe - Nb+Ta (at.%).
Canadian cassiterite contains inclusions of wodginite SnMnTa2O8, which appear lighter than surrounding cassiterite in back-scattered electrons (fig. 1a). In BSE, the concentration gradient in cassiterite matrix in the vicinity of inclusions is visible. The dark zones surrounding inclusions are composed of pure cassiterite - “depleted” cassiterite (depleted in comparison with primary cassiterite, which is enriched in minor elements), whereas the lighter areas farther from the inclusions and inclusion-free are primary “enriched” cassiterite with higher concentration of Ta, Mn, Nb and Fe (tab 1).
|
|
Annie Claim |
Nova Ves |
||||
|
|
IN |
DC |
EC |
IN |
DC |
EC |
|
Ti |
0.025 |
0.000 |
0.000 |
0.151 |
0.015 |
0.010 |
|
Na |
0.012 |
0.084 |
0.059 |
0.000 |
0.069 |
0.044 |
|
Ca |
0.000 |
0.000 |
0.000 |
0.010 |
0.000 |
0.000 |
|
Sc |
0.036 |
0.000 |
0.001 |
0.011 |
0.000 |
0.003 |
|
Sn |
2.274 |
11.928 |
11.059 |
0.089 |
11.786 |
11.157 |
|
Mn |
2.737 |
0.004 |
0.224 |
0.333 |
0.005 |
0.013 |
|
Fe |
0.225 |
0.006 |
0.031 |
3.334 |
0.024 |
0.232 |
|
Zr |
0.402 |
0.012 |
0.042 |
0.086 |
0.051 |
0.034 |
|
Nb |
0.795 |
0.005 |
0.091 |
6.134 |
0.024 |
0.366 |
|
Ta |
5.367 |
0.018 |
0.506 |
1.693 |
0.050 |
0.150 |
|
Y |
- |
- |
- |
0.000 |
0.002 |
0.000 |
|
Th |
- |
- |
- |
0.013 |
0.005 |
0.000 |
|
U |
- |
- |
- |
0.012 |
0.000 |
0.010 |
|
Sb |
- |
- |
- |
0.002 |
0.016 |
0.006 |
|
Hf |
0.077 |
0.006 |
0.008 |
0.010 |
0.006 |
0.004 |
|
W |
0.006 |
0.000 |
0.000 |
0.005 |
0.000 |
0.000 |
|
Bi |
- |
- |
- |
0.000 |
0.000 |
0.000 |
|
Total |
11.956 |
12.062 |
12.022 |
11.883 |
12.052 |
12.029 |
Table 1: Microprobe analyses of
samples 206-IVA from Annie Claim 3 and
MM-116 from Nová Ves.. Calculation is based on 24O. (IN – inclusion, DC – depleted
cassiterite, EC – enriched cassiterite).
Chemical composition of the inclusions
corresponds to wodginite SnMnTa2O6. The major
substitutions are homovalent such as
Chemical composition of the inclusions in cassiterite from the Czech Massif corresponds to columbite Fe(Nb,Ta)2O6. Figure 1c shows cassiterite from Nova Ves in back-scattered electrons. A concentration gradient surrounding inclusion is visible, even though it is much weaker than in the case of samples containing wodginite. In this case, inclusions appear darker than the matrix. The reversed contrast is due to the average molecular weight of inclusions, which is lower than that of cassiterite, whereas in the samples from Annie Claim 3 the inclusions are “heavier” than the matrix. Quantitative analyses are listed in table 1.
The major substitutions active here are homovalent such as Fe-Mn and Nb-Ta. Inclusions concentrate Ti and Nb, while cassiterite prefers Ta. In figure 1d, analyses from sample MM-116 from Nova Ves are plotted in the triangular diagram Sn+Ti - Mn+Fe - Nb+Ta (at.%). Also in this case the analyses fall on the same line, which connects the Sn+Ti apex with (Mn,Fe)(Ta,Nb)2.
STRUCTURAL
ORIENTATION
Cassiterite, SnO2, belongs to the rutile structural group whose members crystallize in space group P42/mnm. Cell parameters of synthetic SnO2 are: a = 4.738 Å, c = 3.186 Å [12]. Its structure consists of edge-sharing SnO6 octahedra that form chains along the c axis and are interconnected in the [110] direction by means of apices of octahedra (fig. 2a).
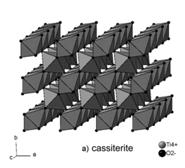
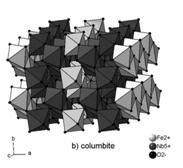
Figure 2:
Structure of cassiterite (a) and columbite (b).
Tantaloniobates such as ixiolite, columbite-tantalite and wodginite, occurring as inclusions in cassiterite have related structures. All theses structures can be described as zig-zag chains of octahedra extending parallel to the c axis (fig. 2b). Individual structures differ by the kind of octahedral cations and their ordering. The structural relationships can be summarized as follows [13]. The common unit is the cell of ixiolite. Ordered columbite-tantalite has unit cell parameters a=3aix, b=bix, c=cix and ordered wodginite a=2aix, b=2bix, c=cix. Therefore, we can expect all these tantaloniobates to assume a similar orientation within the structure of cassiterite.
Structural orientation of tantaloniobate inclusions in cassiterite was studied by means of single-crystal X-ray diffraction and transmission electron microscopy.
X-ray diffraction
The spatial relation of tantaloniobate precipitates within the host cassiterite predicted by a close examination of their structures was confirmed by single-crystal X-ray diffraction. An Enraf-Nonius precession camera with unfiltered MoKa (l = 0.7107 Å) radiation was used. In order to observe diffraction spots of lamellae which represent a small fraction of the volume of host cassiterite, overexposed photographs had to be taken, further boosted with an intensifier screen. Where continuous radiation hampered the interpretation of photographs, the radiation was Zr-filtered.
Mutual crystallographic orientation of the
lattices of cassiterite and tantaloniobate exsolutions (either ferrocolumbite
or wodginite) is as follows (fig. 3): the reciprocal axis c* of each
precipitate is approximately parallel to one of the cassiterite directions
[101]*, [011]*, [![]() ]*, [
]*, [![]() ]*. The precipitates’ a* axes are strictly
parallel to the ±a1* and ±a2* axes of cassiterite, whereas bcol* is not
parallel to any rational reciprocal direction of cassiterite.
]*. The precipitates’ a* axes are strictly
parallel to the ±a1* and ±a2* axes of cassiterite, whereas bcol* is not
parallel to any rational reciprocal direction of cassiterite.
Generally, there are four possible orientations of tantaloniobate precipitates, dictated by the tetragonal symmetry of cassiterite. However, in the crystal from Nova Ves only one orientation was observed (corresponding to col2 in the figure 3b), whereas in sample from Annie-Claim pegmatite at least two different orientations can be observed (fig. 3c).
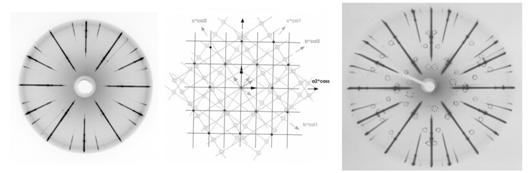
a) b) c)
Figure 3: Precipitates of
tantaloniobates in cassiterite.
(a) precession photograph of 0kl* of cassiterite with 0kl* of ferrocolumbite
(sample MM-116 from Nova Ves -
Electron
diffraction
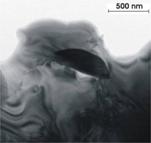
Size, morphology and arrangement of
precipitates and characteristics of interface between host cassiterite and
individual precipitates were studied in TEM at the
Samples were prepared from thin sections by placing Cu-grids on selected areas of interest and ion-milled to the TEM thickness by Ar-ions. Two instruments were used - Philips EM420 at 120 kV and Philips CM300 at 300 kV (FEG), both equipped with an EDS detector. Digital images were treated with Digital Micrograph, and simulation of electron diffraction patterns was produced in MacTempas. EDS analysis were obtained and processed with DTSA and EmiSpec software.
|
|
|
|
|
|
a) |
b) |
c) |
d) |
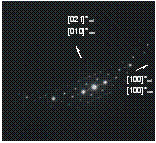
Figure
4:
TEM observations. (a) wodginite inclusion in cassiterite (sample 222a
Annie-Claim pegmatite), (b) corresponding electron diffraction, (c)
ferrocolumbite inclusion in cassiterite (sample MM-97the Czech Massif), (d)
corresponding electron diffraction.
In the TEM (fig. 4a,c), inclusions ranging from 0.X to X mm were observed; no finer scale exsolution products were detected. Chemical composition of the inclusions is the same as analyzed on the microprobe and corresponds to wodginite and ferrocolumbite, respectively.
Structural orientation of the inclusions towards the cassiterite matrix at the interface was examined on about 40 inclusions by means of electron diffraction. The orientation of all the inclusions confirms the results of X-ray diffraction. However, a slight misorientation of several degrees was established.
Examples of inclusions from both localities are shown in figure 4. It can be seen that the reciprocal direction [100]* of cassiterite coincides with the [100]* of inclusions (wodginite or ferrocolumbite). The other parallel directions are: in the sample from Annie Claim [201]* of cassiterite with [010]* of wodginite (fig. 4b) and in the sample from the Czech Massif [021]* of columbite with [100]* of cassiterite (fig. 4d). These relations correspond to the orientation relationships determined by X-ray diffraction (fig. 3b).
CONCLUSIONS
In the samples of cassiterite from the
Annie-Claim Pegmatite (
The mechanism utilized during exsolution of tantaloniobate precipitates in cassiterite is nucleation and growth (not spinodal decomposition – see [14] for description of both mechanisms). The precipitates are in the late stage of exsolution characterized by advanced coarsening and a slight rotation of the phase boundaries. Even though pegmatites are considered to be highly dis-equilibrated and fast cooling rocks [15,16] it seems that at least at some level equilibrium is close to being achieved.
Tantaloniobate precipitates were found in
cassiterite with elevated concentrations of Ta as well as
ACKNOWLEDGEMENTS
We should
like to thank Prof.
REFERENCES
1. P. Černý, F. Čech & P. Povondra, Neues Jb. Miner. Abh. 101(2) (1964), 142-172.
2.
P. Černý, B.J. Paul, F.C. Hawthorne
& R.
3. P. Černý, W.L. Roberts, T.S. Ercit & R. Chapman, Am. Mineral. 70 (1985), 1044-1049.
4. P. Černý, R. Chapman, R. Goad, G. Niedermayer & M.A. Wise, Mineral. Petrol. 40.(1989), 197-206.
5. P. Černý, T.S. Ercit, M.A. Wise, R. Chapman & H.M. Buck, Can. Mineral. 36 (1998), 547-561.
6. P. Černý, R. Chapman, W.B. Simmons & L.E. Chackowsky, Am. Mineral. 84 (1999), 754-763.
7.
P. Černý,
R. Chapman & M. Masau,
8. P. Černý, M. Novák, R. Chapman & M. Masau, J. Czech Geol. Soc. 45/1 (2000), 21-35.
9.
M. Masau,
P. Černý & R. Chapman,
10.
A.M.R.
Neiva.
11.
A.G. Tindle,
F.W. Breaks & P.C.
12. H. Seki, N. Ishizawa, N. Mizutani & M. Kato, J. Ceram. Soc. Jpn. 92 (1984), 219-223.
13.
J.D. Grice,
R.B. Ferguson.& F.C.
14.
A. Putnis: Introduction to Mineral
Sciences.
15.
P. Černý, Geoscience
16.
P. Černý, Geoscience