X-RAY STRUCTURES OF NEW NICKEL COMPLEXES WITH SELECTED S,S-AND N,P-LIGANDS IN THE COORDINATION SPHERE
Jiří Kameníček and Richard Pastorek
.
Department of Inorganic Chemistry, Faculty of Science, Palacký University, 771 47 Olomouc,Czech Republic
Recently, the wide attention was paid to study of nickel coordination compounds with S,S-ligands of the dithiolene type (dithiocarbamates, xanthates, aromatic 1,1-dithiolates, 1,2-dithiolene etc.) due to the many possible practical applications (stabilization of uncommon high oxidation states, modeling of enzymes in biochemistry, pesticides, vulcanization accelerators, flotation agents, high pressure lubricators, superconductors, resins for IR-spectroscopy, pharmacy applications)1-5.
In the lecture, the overview of our last important results will be given. The attention will be focused to the X-ray structure analyses of selected compounds: common nickel complexes with coordination number four (chromophore NiS4, NiS2PX - see Fig. 1, NiS2P2 - see Fig. 2; X = Cl, Br, I, NCS)6,7 and six (chromophore NiS2N4 - see Fig. 3)8. Also the binuclear complexes with bridging P,P-ligand have been synthesized and described9 - see Fig. 4. Moreover, the uncommon complexes with coordination number five (chromophore NiS2P3 – see Fig. 5) and NiS4M – see Fig. 6; M = As, Sb)10,11 have been studied. Finally, the structures of Ni(III) complexes with aromatic 1,2-dithiolene12 - see Fig. 7 have been solved. All structures above will be discussed from structural aspects.
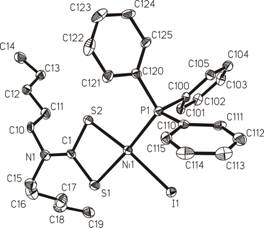 Fig.1. [NiI(dpdtc)(PPh3)] CCDC
197222
Fig.1. [NiI(dpdtc)(PPh3)] CCDC
197222
Fig. 2.
[Ni(dpdtc)(dppf)](ClO4)
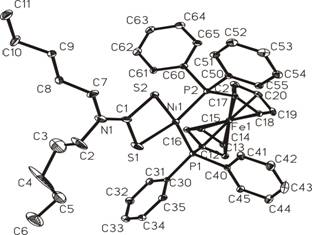
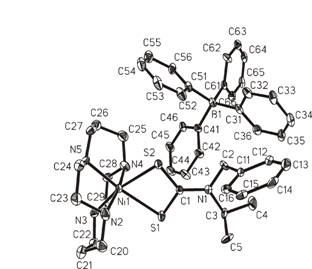 Fig. 3. [Ni(bziprdtc)(cyclam)](BPh4)
Fig. 3. [Ni(bziprdtc)(cyclam)](BPh4)
Fig. 4. [Ni2(m-dpph)(hmidtc)2Br2] CCDC
201015
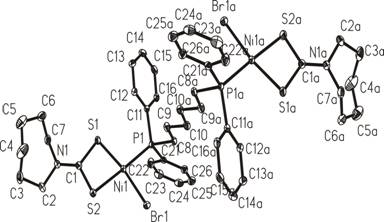
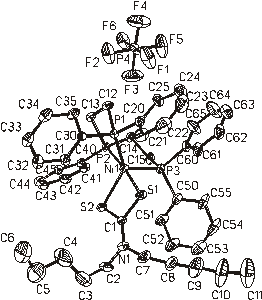
Fig. 5. [Ni(dpdtc)(triphos)](PF6) CCDC
215298
Fig. 6. [Ni(dbdtc)2(SbI3) CCDC
241983
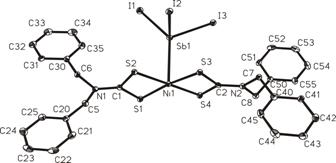
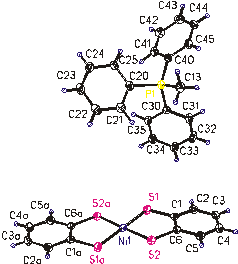
Fig. 7. (MetPPh3)[Ni(bdt)2] CCDC
196004
Conclusions:
All complexes with coordination number four exhibit more or less (depending on type of ligand) distorted square-planar arrangement of coordination sphere around nickel atom; for the compounds with coordination number six, the distorted octahedral polyhedron was found. As to complexes with coordination number five, both possibilities (distorted trigonal bipyramidal - see Fig. 5, or tetragonal pyramidal – see Fig. 6) were confirmed. For the last type of structure (Fig. 7), a significant shortening of Ni-S bonds, corresponding to the assumption of Ni(III) was recorded.
1. J.R. Lancaster: The bioorganic chemistry of
nickel, VCH Publ., Inc. New York 1988.
2. T. Szolnai: Die chemotherapeutischen und
pesticiden Wirkungen der Thiolreagenzien, Akad. Kiadó,Budapest 1975.
3. P. Cassoux, L. Valade, H. Kobayashi, A.
Kobayashi, R.A. Clark, A.E. Underhill, Coord. Chem. Rev. 110
(1991), 115.
4. H. Tanaka, A. Yakoyama, Chem. Pharm.
Bull. 10 (1962), 1133.
5. A. Ogiso, S. Kuroda, N. Ito, Jpn. Kokai
Tokkyo Koho, JP 1045785.
6. R. Pastorek, J. Kameníček, J. Husárek, M.
Pavlíček, Z. Šindelář, Z. Žák, Polish J. Chem. 76 (2002) 1545.
7. R. Pastorek, J. Kameníček, B. Cvek, M.
Pavlíček, Z. Šindelář, Z. Žák: J. Coord. Chem. 56 (2003), 1123.
8. R. Pastorek, J. Kameníček, M. Pavlíček, J.
Husárek, Z. Šindelář, Z. Žák, Acta Univ. Palacki. Olomuc. 40
(2001), 57.
9. R. Pastorek, J. Kameníček, J. Husárek, M.
Pavlíček, Z. Šindelář, Z. Žák, Polish J. Chem. 77 (2003), 805.
10. R. Pastorek, J. Kameníček, J. Husárek, B. Cvek, M. Maloň, M. Pavlíček,
Z. Šindelář, Polish J. Chem. 78 (2004), 623.
11. R. Pastorek, J. Kameníček, Z. Trávníček, M. Pavlíček, B. Cvek, J.
Husárek, Z. Šindelář, Polish J. Chem.
79 (2005), in press.
12. K. Mrkvová, J. Kameníček, Z. Šindelář, L.
Kvítek, J. Mrozinski, M. Nahorska, Z. Žák, Trans. Met. Chem. 29
(2004), 238.