High
pressure phase transition of alcohol intercalated zirconium phosphate
L.
Beneš1, V. Zima1, K. Melánová1, M. Steinhart2,
M. Kriechbaum3, H. Amenitsch3, S. Bernstorff4
1Joint Laboratory of
Solid State Chemistry of Institute of Macromolecular Chemistry of Academy of
Sciences and University Pardubice, Studentská 84, Pardubice, Czech Republic,
2Department of
Physic, University Pardubice, Studentská 84, Pardubice, Czech Republic
3Institute of
Biophysics and X-ray Structure Research, Schmiedlstrasse 6, Graz, Austria
4Sincrotrone Elettra,
Trieste, Basovizza, 34012 Trieste, Italy
Intercalation represents a reversible insertion of mobile atomic or molecular guest species into solid layer host lattice. Intercalates of zirconium phosphate (a-Zr(HPO4)2·H2O, hereafter ZrP) [1] belong among largely studied compounds. In our previous measurements on ZrP-liquid alcohol systems [2], we have observed a phase transition induced by temperature changes. In this phase transition the bimolecular film of alkanols intercalated undergoes a change from an all-trans conformation to a conformation in which the O-C1-C2-C3 torsion angle changes from 180 to 136° during cooling the samples. This leads to the change of the alkyl chain inclination from 59.6° to 47.1°. These two phases differ in their basal spacing. Since the interlayer distance is connected with the thickness of the whole crystal a pressure dependence of the phase behavior can be expected.
Both static and time-resolved measurements were performed at the SAXS channel of the Elettra Synchrotron facility (Trieste, Italy). Further details of the high-pressure system and experimental aspects are given elsewhere [3].
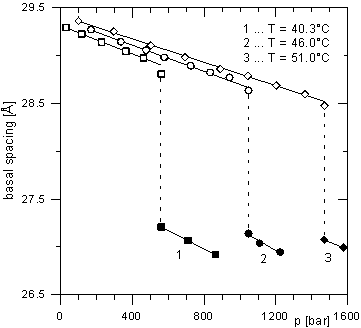
The intercalates of 1-nonanol, 1-octanol and 1-decanol show different behavior. For the 1-nonanol intercalate (see Fig. 1), there is a gradual linear decrease of the basal spacing with increasing pressure at constant temperature up to a certain value of pressure, when a second phase appears. The basal spacing of this second phase also decreases with increasing pressure. Both phases are present in the area of the phase transition. For 1-octanol intercalated ZrP (Fig. 2), the basal spacing vs. pressure dependence is more complicated. At first, this dependence is linear. Just before the phase transition, a small increase of the basal spacing appears. It is followed by a steep decrease of the basal spacing of about 0.8 Å indicating a phase transition. In the intercalates containing a bimolecular film of the guest molecules with aliphatic chains which are not perpendicular to the host layers an even-odd effect can take place [4]. This effect is given by different orientations of the terminal methyl groups of the chains for even and odd numbers of the carbon atoms in the guest chains. Therefore, we can presume that the distinctly different dependence of the basal spacing on pressure in the case of the ZrP intercalate with 1-nonanol compared to the intercalates with 1-octanol and 1-decanol is probably connected with the even-odd effect.
The small increment of the basal spacing with pressure observed for the 1-octanol intercalate is very interesting. It can be caused by steric requirements of the methyl groups during the change of the angle, under which the chains are tilted to the layers, from 59.6 to 47.1°. This phenomenon was not observed for the 1-decanol intercalate, which has, like the 1-octanol intercalate, an even number of the carbon atoms in the chain.
A linear dependence of the phase transition was observed in the p-T diagrams of all three intercalates. The straight lines in the p-T diagrams have almost the same slope for all three alcohol intercalates, but differs distinctly in the temperature, at which the phase transitions occur at ambient pressure.
Acknowledgement
This study was supported by the Grant Agency of the Czech Republic (GV 202/98/K002 and GA 202/01/0520). We would like to express our thanks to Prof. Peter Laggner for providing us with part of his beam-time for our experiment.
References
[1] G. Alberti, U. Costantino in: J. M. Lehn (Ed), Comprehensive
Supramolecular Chemistry, Vol. 7, Pergamon/Elsevier, Elmsford, 1996.
[2] U. Costantino, R. Vivani, V . Zima, L. Beneš, K. Melánova, Langmuir, 18 (2002), 1211-1217.
[3] M. Steinhart, M. Kriechbaum, K. Pressl, H. Amenitsch, P. Laggner, S. Bernstorff, Rev. Sci. Instrum., 70 (1999) 1540-1545.
[4] G. Lagaly, K. Beneke, Colloid. Polym. Sci., 269 (1991) 1198-1211.
Figure 1. Dependence of basal spacing of Zr(HPO4)2·2C9H19OH intercalate on pressure measured at various
temperatures.
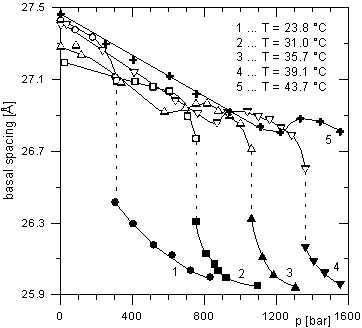
Figure 2. Dependence of basal spacing of Zr(HPO4)2·2C8H17OH intercalate on pressure measured at various
temperatures.