CRYOCRYSTALLOGRAPHY OF BIOLOGICAL MACROMOLECULES
P. Řezáčová1
1Department of Gene
Manipulation, Institute of Molecular Genetics, Academy of Sciences of the Czech
Republic, Flemingovo nam. 2, 166 37 Prague 6, Czech Republic
Introduction
Crystals of biological macromolecules at near
to room temperature are sensitive to X‑rays and frequently suffer from
radiation damage, especially when X-ray experiments are carried out on highly
intense synchrotron beamlines. Performing such experiments at cryogenic temperatures
greatly reduce, or eliminate radiation damage and thus produce higher quality
diffraction data. Since about 1985, the cryocrystallography methods have become
widely used and well established technique. A brief discussion of the most
important experimental aspects and advantages of data collection at low
temperature is given. Reviews on cryogenic techniques in macromolecular
crystallography can be found in: [1, 2, 3].
Radiation
damage
Radiation
damage of biocrystals appears to be related to the formation of free radicals.
Although the photochemical processes producing free radicals (primary radiation
damage) are localized event; subsequent chemical reactions can be induced at
relatively remote sites due to the propagation of free radicals in the solvent
regions of a protein crystal via diffusion (secondary radiation damage) [4].
Damage is spread and leads to crystal decay, typically accompanied by changes
in reflection profiles and cell dimensions.
It
was noted as early as 1970 [5] that performing diffraction experiments on
protein crystals cooled to near liquid-N2 temperature leads to
significant reduction in radiation damage. This effect is due to facts that by
lowering the temperature diffusional processes and therefore propagation of
higly reactive free radicals within the crystals is slowed down.
Experimental
setup
A
schematic of a typical experimental setup of a diffraction experiment at
cryogenics temperature is shown in Figure 1. Instead of being sealed in
capillary, the crystal is mounted in a thin nylon fiber loop and rapidly
cooled, either in a cold gas stream, or by immersion in a cryogen such as
liquid nitrogen. The temperature of the crystal is maintained by a stream of
nitrogen gas during the diffraction measurement. To avoid the formation of ice around the crystal, the nitrogen
stream is shielded agains humidity by a coaxial stream of warm dry air.
Cryoprotection
of crystals
When
a crystal of biological macromolecule is cooled to cryogenic temperature the
main difficulty is to avoid the crystallization of any water present in the
system, whether internal or external to the crystal. Therefore a cooling
procedure has to be chosen that leads to a glass-like amorphous phase of the
solvent [6]. In principle there are three options: (1) cooling on the timescale
too fast for ice formation to occur [7], (2) cooling at high pressure by which
the formation of common hexagonal form of ice is circumvented [8], (3)
modifying the physicochemical properties of the solvent by addition of
cryoprotectants in a way that vitrified state can be reached at moderate
cooling rates. The latter method is currently the most widely used. A list of
cryoprotectants used successfully with macromolecular crystals is shown in
Table 1 [9]. Glycerol appears to be a widely applicable cryoprotectant and
is frequently chosen for initial trials [10]. Typically cryoprotectants are
included in the established harvesting solution at concentration range 50%.
Methods for introducing the cryoprotectants are: (1) serial transfer into
increasing concentrations of cryoprotectant, (2) dialysis, (3) growth in
cryoprotectant, (4) brief transfer before flash cooling, (4) direct transfer
into full strenght cryoprotectant. It is rare that crystal can be transferred
without damage directly to a solution containing full-strenght cryosolvent.
Usually, the cryoprotectant must be introduced slowly to reduce stress on the
crystal lattice. Finding suitable cryoprotection conditions is a
trial-and-error process. Two problems must be overcome: the cryoprotectant must
be introduced without significant damage to the crystal, and damage during the
flash cooling must be minimized.
Crystal
mounting and data collection
To
facilitate rapid heat transfer the crystal must be in immediate contact with
the cooling medium and therefore capillaries cannot be used for mounting.
Depending on the mechanical properties of the crystal, glass fibres, glass
spatulas or, currently most widely fibre loops are used [11]. Using the nylon
fibre loop, protein crystal is picked up by swiftly moving the loop alongside
the crystal from the crystallization mother liquor. The crystal is held within
the film by surface tension and after equilibration in cryoprotective buffer
must be cooled to cryogenic conditions as soon as possible. A simple and often
effective approach is to flash cool the crystal in a goniostat nitrogen stream
right on the X-ray camera. This technique has the added advantage in leaving
the crystal in position for immediate analysis and data collection. An
alternative method, rapidly plunging the crystal into a liquid cryogen, also
offers several advantages. It reduces the time between mounting the crystal and
flash cooling, it produces higher cooling rate and results in more even cooling
of both sides of loop-mounted sample. Crystal flash cooled in a liquid nitrogen
must be placed for data collection in the cold gas stream a goniostat without any substantial warming.
Storage
and transport of crystals
Once
a crystal has been successfully cooled to cryogenic temperature it can be in
principle stored for indefinite time. This allows to cool and characterize
crystals on a conventional source in the home laboratory and then store them
until synchrotron time becomes available. Dewars that can be used for
transport, including shipment by airplane, are available.
Conclusion
and perspectives
Cryogenic
methods provide great advantages in macromolecular crystallography especially
when synchrotrone radiation is used for diffraction data collection. Apart from
eliminating the problem with radiation damage and enabling the storage and safe
transport of frozen crystals, there are number of additional benefits. In
general higher quality data can be achieved and in many cases all data can be
collected from a single crystal. Cryogenic data collection has allowed
efficient phasing using multiwavelength methods. Additionally, a crystal at
cryotemperature is rigidly attached to its mount, so that slippage during the
measurement is impossible.
1.
H.
Hope, Annu. Rev. Biophys. Chem. 19 (1990) 107
2.
K.
D. Watenpaugh, Curr. Opin. Str. Biol. 1 (1991) 1012
3.
D.
W. Rodgers, Structure 2 (1994) 1135
4.
C.
Nave, Radiat.Phys. Chem. 45 (1995) 483.
5.
D.J.
Haas, M.G. Rosmann, Acta Cryst. B51 (1970) 998.
6.
T.
Y. Teng, J. Appl. Cryst. 23 (1990) 387
7.
H. Hartmann, F. Parak, W. Steigemann, G. A. Petsko, D. Ringe-Ponzi, H.
Frauenfelder, Proc. Natl. Acad. Sci. USA 79 (1982) 4967
8.
U.
F. Thomanek, F. Parak, R. L. Mossbauer, H. Formanek, P. Schwager, W. Hope, Acta
Cryst. A29 (1973) 263
9. D. V. Rodgers, in International Tables for Crystallography
Vol. F
(2001) 202
10.
E.
F. Garman, E. P. Mitchell, J. Appl. Cryst. 29 (1996) 584
11.
T.-Y.
Teng, J. Appl. Cryst. 31 (1998) 252
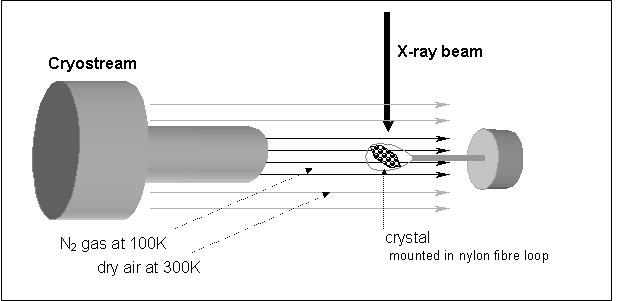
Figure 1. Schematic view of a typical
experimental setup of diffraction experiment at cryogenics temperature
Table 1.
|
List of cryoprotectans used successfully for flash
cooling of biological crystals |
|
|
Erythriol |
2-Metyl-2, 4-pentanediol (MPD) |
|
Ethanol |
Polyethylen glycol 400 |
|
Ethylen glycol |
Polyethylen glycol 1000 – 10 000 |
|
Glucose |
Propylene glycol |
|
Glycerol |
Sucrose |
|
Methanol |
Xylitol |