CRYSTALLIZATION OF BIOLOGICAL
MACROMOLECULES
Ivana Kutá Smatanová
Institute of Physical Biology, University of South Bohemia, 373 00 Nové Hrady, The Czech Republic
All of the macromolecules
are polymers of one of the precursor classes that include the amino acids, the
ribonucleotides and deoxyribonucleotides, sugars of various sorts, fatty acids,
etc. These small molecules are linked together in a sequence by complicated
series of chemical reactions in the cell to form the macromolecules such as proteins,
nucleic acids (RNA and DNA), polysaccharides and lipids. The structural
complexity and physiological role of macromolecules are a function of the
diversity of the precursors, the sequence in which they are joined together,
the number of precursors in the polymer, and finally, the 3D form after polymer
synthesis. Macromolecules assume 3D structures that sequester and pack
hydrophobic groups in their interior and leave hydrophilic groups exposed to
solvent. Solvent molecules form solvent layers around macromolecules. In this
abstract, we are concerned mainly with properties and crystal structures of
proteins.
Protein crystals contain
network of solvent filled channels that make up 40-80% of their volume. Due to
the high solvent content as well as the limited number of weak interactions
that hold the crystal together, the environment of the molecules in the crystal
resembles that of a very concentrated solution.
The first step in the
crystallization of macromolecules is their purification
and characterization. Some proteins
form precipitates at low salt concentration, others only from highly
concentrated salt solutions, and in some cases, only when salt is removed from
solution. Because proteins precipitate at different salt concentrations, this
“salting-out” effect provides a method for selectively precipitating and
purifying unique proteins from mixture. In recent years, polymeric
precipitating agents (Peg in variety of polymer lengths) as well as organic
solvents (ethanol, acetone, MPD) have been used to selectively precipitate
macromolecules. The most common buffers are intended to be effective in the pH
range of 6.0 to 8.0, because the most physiologically important reactions occur
near neutral pH. For specific pH ranges, biological buffers extending from pH 2
to 13 are used. The similar conditions as for purification and isolation of
proteins are used to provide following crystallization experiments.
The solubility of proteins
in water depends on properties such as temperature, pH, and the presence of
other solution components as well as amino acid composition. When the
concentration of a protein solution is brought above its solubility limit [see
figure 1], the solution becomes supersaturated. At this point, the protein
begins to aggregate. Aggregation occurs in two stages as a nucleation and as a
growth. During nucleation, protein molecules associate into a stable complex as
an amorphous precipitate or as a microcrystals. Amorphous precipitates tend to
predominate when the protein concentration is well above saturation. In
addition, crystals grow much slower than amorphous precipitates do, so when the
concentration of a protein is brought above its saturation point too quickly,
precipitation will again predominate. In the metastable region, if a few nuclei
are present, they will continue to grow, but without spontaneous formation of
new nuclei. As the crystals grow, the solution will be depleted of nutrient and
supersaturation will fall. Growth will be slow and orderly and will produce the
fewest and largest single crystals. Thus, the most general crystallization strategy is to bring the protein to the point
only slightly above its saturation point as slowly as possible.
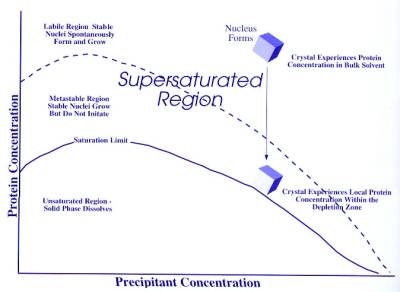
Figure 1. The phase diagram for the description of protein crystallization (from McPherson, 1999)
Generally, the protein crystallization experiments proceed in two steps. First step is test screening of the protein solubility considering the precipitants (precipitating agents) and other solution components. Usually at this step, insoluble protein is observed as an amorphous precipitate, what means that the precipitation conditions are severe to allow crystal growth. In the second optimization step, conditions which gave rise to precipitates in the first step are modified systematically to allow the approach to insolubility that is required for the formation of crystal nuclei.
Several techniques such as
sitting drop vapor diffusion, hanging drop vapor diffusion, sandwich drop,
batch, microbatch under oil, microdialysis, and free interface diffusion could
be used for setting up crystallization experiments. The most frequently used
crystallization method is the vapor
diffusion technique. The difference in concentration between the drop
(protein, buffer, salt and precipitant) and the reservoir (buffer, salt and
precipitant) drives the system toward equilibrium by diffusion through the
vapor phase. The protein becomes supersaturated and crystals start to form when
the drop and reservoir are at or close to equilibrium. Free interface diffusion is one of the methods used by NASA in
microgravity crystallization trials. Using this method the sample is in liquid
contact with the precipitant. Over time the sample and precipitant diffuse into
one another and crystallization may occur at the interface. Batch
crystallization is method where the sample is mixed with the precipitant
and additives creating a homogenous crystallization medium. This technique is
popular with small molecule crystallographers. In microbatch under oil a small drop of the sample combined with the
crystallization agent is pipetted under a layer of oil (paraffin, silicon
oils). Such oils allow water vapor to permeate from the drop and allow sample
and reagent concentration. Unless the drop is equilibrated with a reservoir, water
will leave the drop until those only solids remain. Dialysis crystallization involves placing the sample in a Dialysis
Button, which is sealed with a dialysis membrane. The Dialysis Button is placed
into a container with crystallization medium. Water and some precipitants are
then allowed to exchange while retaining the sample in the cell.
For the examination of the crystallization trials a stereomicroscope is
used. Crystals are usually easy to distinguish from amorphous precipitate.
Diffractable crystals are typically single, transparent, they have definite
form characterized by planar faces and they are free of cracks and defects.
Crystals are often birefringent, so that they appear dark and bright as they
are rotated under crossed polarizers in the stereomicroscope. Several methods
are available to test whether crystals are protein or salt. These are crush
test, dehydration test, dye binding test, gel electrophoresis and X-ray
diffraction. In the case of getting microcrystals, the seeding techniques could
be used to grow the crystal. The seeds (microcrystals) are transferred to a new
protein-precipitant drop using a streak seeding wand or a crystal transfer
syringe, respectively. Seeds provide a template on which further molecules can
assemble, and given the proper environment, time, and patience, the seed will
enlarge into a crystal.
The crystallization of
membrane proteins proceeds in the same manner as crystallization of soluble
proteins, except for the addition of detergents in the crystallization
conditions. Selection of proper detergent is the most critical parameter for
this kind of crystallization.
Experience and reproducibility are guides in making
crystallization experiments.
A. McPherson:
Crystallization of Biological Macromolecules. New York 1999. CSHL Press.
T. M. Bergfors: Protein
Crystallization: Techniques, Strategies and Tips. La Jolla 1999. IUL
Biotechnology Series.
E. Fanchon et al.: Structure
and Dynamics of Biomolecules. Oxford 2000. Oxford University Press.
D. M. Bolag et al.: Protein
Methods. New York 1996. Wiley-Liss, Inc.
Hampton Research: Crystallization: Research Tools, Vol.
11, No. 1 (2001) 152-165.