Three epochs of nascent protein translocation through the ribosome exit tunnel
M. H. Kolář, H. Mc Grath, F. C. Nepomuceno
Department of Physical Chemistry, University of Chemistry and Technology, Technická 5, 166 28 Prague
kolarh@vscht.cz
All proteins in living organisms are produced in ribosomes that facilitate the translation of genetic information into a sequence of amino-acid residues. During translation, the ribosome undergoes stages of initialization, elongation, termination, and recycling. In fact, peptide bonds are formed only during the elongation stage, which comprises periodic association of transfer RNAs and multiple auxiliary proteins to the ribosome, and adding an amino acid to the nascent polypeptide one at a time.
Protein spends a considerable amount of time attached to the ribosome. In this context, we conceptually divide the con-translational lifespan of protein into three epochs. We define the epochs on the basis of the position of the N-terminus of the nascent polypeptide within the ribosome exit tunnel and the context of the catalytic center.
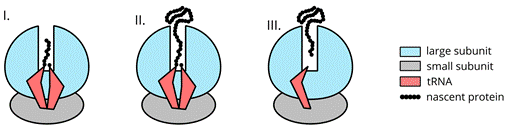
Figure 1: A schematic representation of the three epochs of nascent protein translocation.
In the first epoch, the N-terminus of the nascent protein travels from the catalytic site of the ribosome towards the tunnel exit. During the second epoch, the N-terminal part of the nascent protein remains outside the ribosome and typically undergoes co-translational folding. In the third epoch, the C-terminus is cleaved off the tRNA, escaping from the ribosome exit tunnel.
In the talk, we argue that nascent proteins experience a variety of forces that determine how they translocate through the tunnel and interact with the tunnel walls. We review current knowledge about translocation and identify several gaps in our understanding of the birth of proteins.
The research was supported by the Czech Science Foundation (project 23-05557S).