Electron Transport Mechanisms in Protein Junctions Investigated by Computational Techniques Based on Density Functional Theory
Zdenek Futera
Faculty of Science,
University of South Bohemia, Branišovská 1760,
370 05 České Budějovice, Czech Republic
Electron transfer facilitated by redox-active proteins is utilized in various biological processes, including photosynthesis, respiration cycle, or denitrification reactions. Blue copper proteins such as Plastocyanin or Azurin and the heme-containing cytochromes often participate in these redox cascades. Recently, these proteins started to be utilized in nanobioelectronic devices due to their suitable electron-transfer properties. However, non-expected physical phenomena were observed when the proteins were incorporated between metal contacts or electrodes. While in a native aqueous environment, the electron flow through the system of redox sites proceeds by the thermally activated hopping mechanism, the temperature-independent currents of relatively high magnitudes were detected on protein/metal junctions.1,2 These data suggest that the electrons on the bio/metallic interfaces and junctions are transferred by the coherent tunneling mechanism, independently of the redox-active states.
We investigate these electron-transport phenomena by means of computer simulations based on classical molecular dynamics (MD) as well as the first-principles description within the framework of density functional theory (DFT).3,4 While the incoherent hopping could be studied by combined quantum-mechanical/molecular-mechanical (QM/MM) techniques,5 the coherent tunneling requires a quantum description of the whole interface models. Recently, we applied these methodologies on Azurin blue-copper protein and on small tetraheme cytochrome (STC), which were previously studied experimentally. We showed that the transport mechanism in both Azurin and STC junctions between gold electrodes is the coherent tunneling facilitated by valence-band states of the proteins. In contrast to their redox properties in solution, the presence of the metal cations in the protein structures is not essential for their conductivity on the metal interfaces. The reason for this drastically different behavior in solution and on the metal interfaces is the significant electronic-level misalignment between the protein and metallic states.6
 (a)
(a) 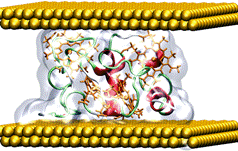 (b)
(b)
Figure 1: Structure of (a) the blue-copper protein Azurin, and (b) STC gold junctions.