Crystallization and binding characterization of recombinant variants of rhesus cytomegalovirus glycoprotein UL144
A. Bitala, M. Benko, S. Lenhartová and I. Nemčovičová
Biomedical Research Center, Institute of Virology, Slovak Academy of Sciences, Bratislava, Slovakia
Isoforms of CD160 interact with ligands through the immunoglobulin superfamily (IgSF) domain, including modest interactions with classical and nonclassical class I molecules of the major histocompatibility complex (MHC). Herpes virus entry mediator (HVEM), a known member of the tumour necrosis factor (TNF) receptor superfamily (TNFRSF), is one of the binding partners of CD160. CD160 and HVEM are the key regulators that exhibit multiple functional outcomes, including suppression of CD4+ T cell proliferation, interferon-γ (IFN-γ) production, increase cytokine production, promotion of lytic activity in NK cells [1, 2] and many others. Transmembrane glycoprotein UL144, encoded in the UL/b' genomic region of Human Cytomegalovirus (HCMV), is additionally homologous to HVEM. UL144 plays an important role in virus entry into the host cell using principles associated with HVEM. However, comparative studies revealed the ability of HVEM to activate NK cells to a higher degree than its viral counterpart UL144, which reflects the inability of UL144 to bind CD160. Interestingly, UL144 isolated from Rhesus Cytomegalovirus (RhCMV) interacts with both human and the rhesus CD160 with low affinity [3, 4] that represents evolutionary divergence between viral species.
We have further investigated into CD160 function and recombinantly prepared mutant UL144 that lacks all glycosylation sites. The binding to CD160 was observed with affinity comparable to that of soluble HVEM. It emphasizes the importance of post-translational modifications, including glycosylation. Moreover, the binding formation was observed by using co-immunoprecipitation assay (co-PI) between recombinant His-tagged UL144 variants (e.g., RhUL144-WT, UL144-WT, UL144-DG, UL144-N91) and human Fc-tagged CD160. To fully understand the molecular-structural basis of these interactions, more specific analyses will be completed in the future. In addition, we have performed the crystallization condition screening for all variants of UL144 alone (Fig. 1). Preliminary crystallization conditions were found and are being further optimized. The suitable crystals have been tested for X-ray diffraction. The structural data of all protein crystals are being optimized and processed for phasing.
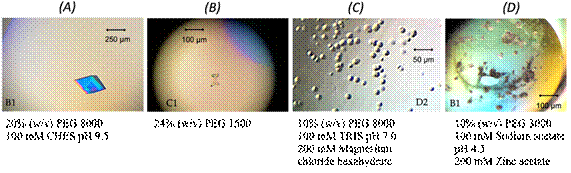
Figure 1. The crystals of RhCMV UL144-WT (A, B); HCMV UL144-DG (C) and HCMV UL144-N91 (D) were obtained under above-mentioned crystallization conditions with precipitant to protein ratio 1:2 in the drop.
1. G. Cai, and G. J. Freeman, Immunol Rev 229, (2009), 244–258.
2. M. Maeda, and M. C. Carpenito, J Immunol 175, (2005), 4426–4432.
3. A. Bitra, and I. Nemčovičová, JBC 294, (2019), 10519–10529.
4. J. R. Šedý, and R. L. Bjordahl, J Immunol 191, (2013), 828–836.
This research was funded by the contribution of the Slovak Research and Development Agency under the project APVV-19-0376 and the contribution of the Scientific Grant Agency of the Slovak Republic under the grant VEGA-02/0026/22.