RimM and RsfS ribosomal factors regulate the translation in bacteria
Ahmed H. Hassan1, Yuko Nakano2, Gregor Blaha3, Ya-Ming Hou2 and Gabriel Demo1
1Central European Institute of Technology, Masaryk University, Brno, Czech Republic.
2Department of Biochemistry and Molecular Biology, Thomas Jefferson University, Philadelphia, USA.
3Department of Biochemistry, University of California, Riverside, California, USA
E-mail: Ahmed.hassan@ceitec.muni.cz
It is crucial for bacteria to save energy when stressed or the access to nutrients is limited. Since synthesis of proteins is an important energy draining source, bacteria rely on slowing down the protein synthesis in harsh conditions [1]. The ribosomal silencing factor (RsfS) has a significant role in stalling the protein synthesis by binding the 50S ribosomal subunit and inhibiting the assembly of 70S ribosome [2]. Meanwhile, the ribosomal maturation factor RimM is vital for the maturation of the 30S small ribosomal subunit. Moreover, during stress RimM is highly enriched in the hibernating 100S dimerized ribosome to stall the protein synthesis and preserve energy [3]. In this work, we use a combination of biochemical studies and cryo-electron microscopy to elucidate the close relationship between RimM and RsfS, and their regulation capabilities on translation.
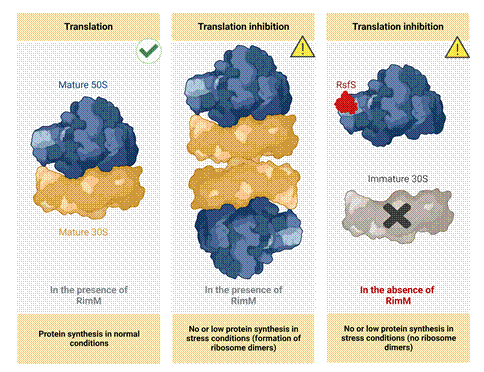
Figure 1. In normal conditions, bacteria with intact 70S ribosomes perform regular protein synthesis. Under stress or nutrients deprived conditions, bacterial 70S ribosomes in presence of RimM dimerize to 100S to stall protein synthesis. Deletion of RimM results in accumulation of RsfS to inhibit the assembly of 70S ribosome.
1. Starosta AL, et al. The bacterial translation stress response. FEMS Microbiol Rev. 2014; 38(6):1172-201.
2. Li X, et al. Structure of ribosomal silencing factor bound to Mycobacterium tuberculosis ribosome. Structure. 2015; 23(10):1858-1865.
3. Suzuki S, et al. Structural characterization of the ribosome maturation protein, RimM. J Bacteriol. 2007; 189(17):6397-406.
This study was supported by LL2008 project with financial support from MEYS CR as a part of the ERC CZ program (to G.D.).