Thermo Scientific™ Tundra Cryo-TEM: 100kV Cryo-TEM dedicated for Single Particle Analysis
Zuzana Hlavenkova1, Dimple Karia2, Milos Malinsky1, Daniel Nemecek1, Fanis Grollios2, Vojtech Dolezal1, Ondrej Shanel1, Abhay Kotecha2, Marketa Cervinkova1, Lingbo Yu2
1 Thermo Fisher Scientific Brno s.r.o., Materials & Structural Analysis, Brno, Czech Republic
2 Thermo Fisher Scientific, Materials & Structural Analysis, Eindhoven, Netherlands
Single Particle Analysis (SPA) application of cryo-electron microscopy (cryo-EM) has become a widely used method for determination of the 3D structure of broad types of proteins and protein complexes, to study the mechanism of their function[1]. As the popularity of this technique increases, so does the need for greater efficiency and accessibility from not only microscopy experts but also from broader audience with little to no cryo-EM experience.
The Thermo Scientific Tundra Cryo-TEM is a new transmission electron microscope operating at 100kV acceleration voltage dedicated to SPA[2] which has been developed especially for new Cryo-EM users.
To load the sample into the microscope, Tundra Cryo-TEM brings a novel semi-automated loading technology (SAL). Sample loading operation is supported by fully guided workflow on the on-screen display (OSD) and in a few minutes allows to load the vitrified sample to the TEM column for people with very limited Cryo-EM expertise.
For 100kV accelerate voltage a new scintillator-based camera CETA-F with speed enhancement was developed. CETA-F is dedicated for low dose application and brings the possibility of dose fractionation mode as is implemented on the Falcon camera. This allows to store image frames for correction of beam induced motion in a post processing pipeline.
A new objective lens was developed for Tundra Cryo-EM to decrease the spherical and chromatic aberrations at 100-kV acceleration voltage and boost signal at high resolution frequencies.
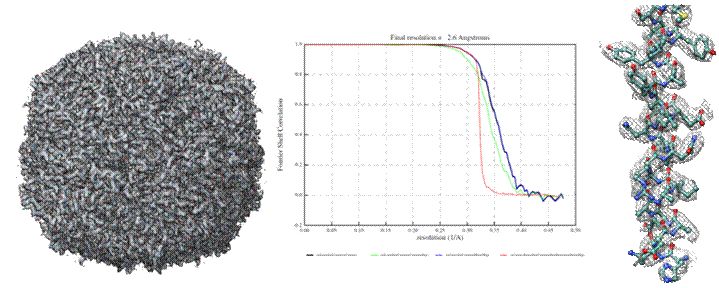
By using all these new features Tundra Cryo-TEM achieved using apo-ferritin 2.6Å resolution of a reconstructed 3D map (Figure 1). At this resolution, de novo protein structures can be determined, and important biological questions answered. Data was collected with pixel size of 0.75Å, using aberration free image shift (AFIS) technology for less than 4 hours and processed using Relion 3.1[3]. The data was collected using Thermo Scientific™ EPUTM software with pre-defined settings, were a new functionality in EPU was used – to automatically checks and refines optical alignments and provides system status for high-quality data acquisition.
All these new features that are introduced within the Tundra Cryo-TEM to achieve relevant resolution of their biological samples while keeping an accessible price point. This would make cryo-TEM accessible to many scientists across all life science branches.
[1] Michael Eisenstein: The field that came in from the cold, Nature, Vol.13 No.1, January 2016
[2] Mathew J. Peet, Richard Henderson, Christopher J. Russo: The energy dependence of contrast and damage in electron cryomicroscopy of biological molecules, Ultramicroscopy 203 (2019) 125–131
[3] J. Zivanov, T. Nakane, B. Forsberg, D. Kimanius, W.J.H. Hagen, E. Lindahl & S.H.W. Scheres "RELION-3: new tools for automated high-resolution cryo-EM structure determination", eLife 2018;7:e42166
|
a) |
b) |
c) |
Fig.1 Structure of Apoferritin protein determined at 100 keV. a) 3D reconstruction of apoferritin at 2.6Å resolution, b) Gold-standard FSC plot corresponding to the calculated map, showing the correlation between the phase-randomized (red), unmasked (green) and masked (blue) half-maps, c) Electron density of the 2.6 Å resolution map showing the apoferritin α-helix