Structural and Biophysical Aspects of Lactoferrin and Its Interaction with Plasminogen
Patricia Hrasnova, Klaudia Meskova, Rostislav Skrabana
Institute of Neuroimmunology, Slovak Academy of Sciences
patricia.hrasnova@gmail.com
Recently it was shown that lactoferrin (Lf), immunomodulatory protein of transferrin family of proteins interacts with plasminogen (Plg), protein of the fibrinolytic cascade [1]. The most probable mode of interaction is the contact of the N-terminal domain of Lf (corresponding to its peptide lactoferricin) with a mini-Plg region consisting of kringle 5 and a proteolytic domain.
Our aim is to further characterise the properties of Lf, comparing its recombinant and natural form. Unlike the native Lf, commercially available recombinant Lf produced in transgenic rice bears different type of glycosylation. Therefore, we aimed to prepare a recombinant Lf with human-like glycosylation by its expression in mammalian CHO cell line.
Depending on the environment, Lf may oligomerise, losing its bactericidal and fungicidal immunomodulatory characteristics [2, 3]. Some properties of Lf can be changed in the presence of divalent ions (especially calcium ions [4]) inducing aggregation into higher oligomeric states. We used dynamic light scattering analysis to characterise Lf oligomerisation.
Previous results indicated that the functional part of Lf interaction with Plg consists of the peptide lactoferricin [1]. Our next goal was to optimise its production by pepsin digestion and its subsequent isolation. To determine the exact interaction of prepared lactoferricin with Plg, affinity determination by surface plasmon resonance will be used. Additionally to these experimental approaches, in silico molecular docking characterising the interaction sites will be applied.
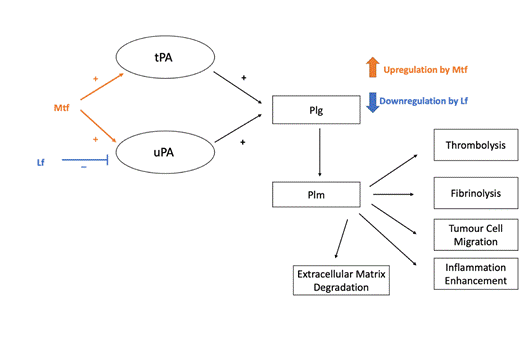
Figure 1: Plasminogen (Plg) activation to plasmin (Plm) by tissue-type plasminogen activator (tPA) and urokinase plasminogen activator (uPA) (modified from [5]) and its regulation by melanotransferrin (Mtf) and lactoferrin (Lf ).
1. A. Zwirzitz, M. Reiter, R. Skrabana, A. Ohradanova-Repic, O. Majdic, M. Gutekova, O. Cehlar, E. Petrovčíková, E. Kutejova, G. Stanek, H. Stockinger, & V. Leksa, J. Biol. Chem., 293, (2018), pp. 8600–8613.
2. R. T. Ellison Ellison, F. M. LaForce, T. J. Giehl, D. S. Boose, & B. E. Dunn, J. Gen. Microbiol. 136, (1990), pp. 1437– 1446.
3. M. Viejo-Díaz, M. T. Andrés & J. F. Fierro, Antimicrob. Agents Chemother., 48, (2004), pp. 1242–1248.
4. G. C. Bagby & R. M. Bennett, Blood, 60, (1982), pp. 108– 112.
5. M. P. Tsantarliotou, S. N. Lavrentiadou, I. A. Zervos, A. N. Kokoli &
I. A. Taitzoglou,. Small Rumin. Res., 76, (2008), pp. 120–130.