Characterize your most challenging interactions. The New Monolith.
Piotr Wardega, Pawel Kania
NanoTemper Technologies sp.z o.o. Kraków, Poland
pawel.kania@nanotempertech.com
Knowing the strength of the interactions between key players is crucial to get the insights you need to understand the details behind how a given biological event occurs.
The new Monolith is the latest solution we provide to our customers who wish to quantify their biomolecular interactions of any kind in any experimental conditions. Monolith utilizes two proprietary technologies- MST as well as our newest addition to the portfolio- isothermal spectral shift.
MST technology allows for quantification of molecular interactions between a target and ligand by detecting changes in fluorescence intensity while a temperature gradient is applied over time. The fluorescent signal comes from the target that is either fluorescently labeled or has intrinsic fluorescence and becomes an extremely sensitive reporter for the interaction.
A
Isothermal spectral shift in order to quantify a
molecular interactions utilizes an experimental procedure during which a fluorescently
labelled target generates a particular emission spectrum, and if a ligand binds
to this target, the fluorophore’s local chemical environment is changed,
causing a shift in its fluorescence spectrum. This particular Monolith detector
exploits this phenomenon by performing ratiometric measurements at two emission
wavelengths of a labelled target in the presence of various concentrations of a
ligand.
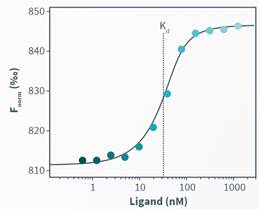
In both of the detectors types which can be combined in a Monolith instrument- the binding affinity is automatically determined at the end of each run without additional and lengthy data analysis. (figure 1.)
Figure 1. The affinity constant (Kd) is calculated from a fitted curve that plots normalized fluorescence against concentration of ligand.
B
Monolith enables characterization of in solution
interactions for a wide range of biomolecules, even for challenging samples
such as membrane proteins, intrinsically disordered proteins, small molecules and
cell lysates. Since the binding partners are in solution, there is no lost activity
due to immobilization, and evaluation is size independent. Measurements can be
performed in any buffer, including detergents, using low sample volumes and
concentrations. The collected data analysis also facilitates the evaluation of
competition assays and ternary binding events.
Monolith provides a valuable orthogonal method to validate your results from other biophysical methods and to characterize your most challenging interactions.
A)
 B)
B)