Designing transmembrane proteins to enhance transport of peptides across cell membranes
L. Bartoš1,2, R. Vácha1,2,3
1Central European
Institute of Technology (CEITEC), Faculty of Science, Masaryk University,
Kamenice 735/5, Brno, Czech Republic
2National Centre for Biomolecular Research, Faculty of Science,
Masaryk University, Kamenice 735/5, Brno, Czech Republic
3Department of Condensed Matter Physics, Faculty of Science, Masaryk
University, Kotlářská 267/2, Brno, Czech Republic
e-mail: robert.vacha@mail.muni.cz
Cell membranes are multicomponent semipermeable bilayers providing selective transport of molecules between the cell and its surroundings. Only certain classes of molecules are able to pass directly through the hydrophobic core of the membrane, the transport of other molecules can be catalyzed by transmembrane proteins. One of such proteins are scramblases which facilitate the translocation of lipids between individual membrane leaflets [1].
We hypothesize that scramblases and proteins with similar properties could also enhance membrane translocation of other amphiphilic molecules, such as antimicrobial or cell-penetrating peptides [2, 3].
Using coarse-grained molecular dynamics simulations with free energy calculations, we systematically study and identify properties of transmembrane proteins leading to maximal enhancement of peptide translocation across phospholipid membranes. We show that the optimal translocation-enhancing proteins contain i) hydrophilic residues forming continuous and compact patch, ii) charged residue(s), preferably positioned in the protein center, and iii) large aromatic residues. Furthermore, we reveal that the translocation enhancement originates from i) membrane disruption caused by the protein and ii) stabilizing enthalpic interactions between the protein and the translocating peptide.
Our results demonstrate that naturally occuring scramblases or de novo designed proteins or peptides could be used for more efficient transport of amphiphilic peptides into cells. This opens the possibility of developing a drug-delivery system by mixing a peptide-based drug with translocation-enhancer that would integrate into the membrane.
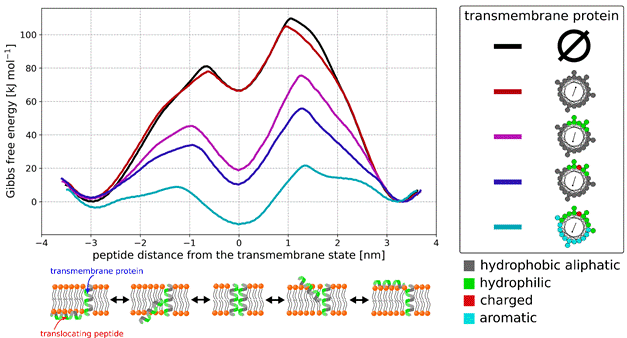
Figure 1. Free energy profiles of a selected amphiphilic peptide translocating through the membrane along various transmembrane proteins. As the translocation-enhancing properties of the transmembrane protein increase, the free energy barrier of peptide translocation decreases. The mechanism of peptide translocation along the transmembrane protein is schematically depicted below the chart.
1. H. M. Hankins, R. D. Baldridge, P. Xu, T. R. Graham. Traffic, 16, (2015), 35-47.
2. J. Wang, X. Dou, J. Song, Y. Lyu, X. Zhu, L. Xu, W. Li, A. Shan. Med Res Rev., 39, (2019), 831-859.
3. G. Guidotti, L. Brambilla, D. Rossi. Trends Pharmacol Sci., 38, (2017), 406-424.
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No 101001470) and from the Czech Science Foundation (project no. GA20-20152S). Computational resources were provided by the CESNET LM2015042 and the CERIT Scientific Cloud LM2015085 provided under the program Projects of Large Research, Development, and Innovations Infrastructures. Additional computational resources were obtained from IT4 Innovations National Super-computing Center – LM2015070 project supported by MEYS CR from the Large Infrastructures for Research, Experimental Development and Innovations.