Raman Optical Activity of Nucleotides – Theoretical and Experimental Study
V. Schrenková1,2*, J. Kessler2, and P. Bouř1,2
1Department of Analytical Chemistry, University of Chemistry and Technology, Technická 5, Dejvice, 166 28 Praha 6
2Institute of Organic Chemistry and Biochemistry, Flemingovo náměstí 2, 166 10 Praha 6
vera.schrenkova@uochb.cas.cz
Biological activity of nucleotides is strongly dependent on their conformation. For example, chemically modified (oligo)nucleotides are widely applied as therapeutical agents and alternation in the structure impacts their conformational dynamics.1 Raman optical activity (ROA) can be conveniently used to examine the conformation of biomolecules in aqueous solutions. However, ROA applications to nucleotides are rather scarce due to the complexity of the experiment and calculations. To investigate the potential of this spectroscopy, Raman and ROA spectra of common mononucleotides (rAMP, rGMP, rCMP and dTMP) were measured and interpreted on the basis of molecular dynamics combined with density functional theory. It was shown, for example, that the sugar puckering in all nucleotides is strongly dependent on intramolecular H‑bonding. Further analysis of theoretical free energy surfaces as well as simulated and experimental spectra revealed details of the conformer distribution.
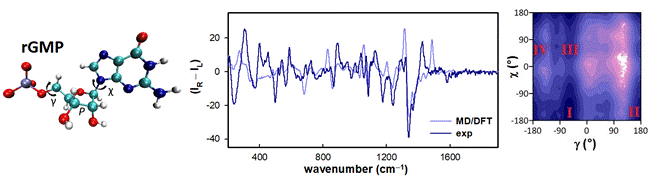
Figure 1. Structure of rGMP (left), calculated and experimental ROA spectra (middle) and dependence of the free energy on the χ and γ torsion angles as obtained from molecular dynamics (right).
1. Evich M., Spring-Connell A. M., Germann M. W.: Heterocyclic Communications 23, 155 (2017).