Exploring Reaction Mechanisms of Metalloproteins by Correlating Theory and Experiment
L. Rulíšek1, M. Srnec2, D. Bím1,2,3, J. Chalupský1, E. I. Solomon4
1Institute of Organic
Chemistry and Biochemistry, The Czech Academy of Sciences, Flemingovo nám. 2,
Prague 6 166 10;
2J. Heyrovský
Institute of Physical Chemistry, The Czech Academy of Sciences, Dolejškova 3,
Prague 8 182 23, Czech Republic;
3Division of
Chemistry and Chemical Engineering, California Institute of Technology,
Pasadena, California 91125.;
4Department of Chemistry, Stanford
University, 333 Campus Drive, Stanford, California 94305-5080, U. S. A.
rulisek@uochb.cas.cz
Among the various essential elements in biocatalysis, metalloproteins play a specific role by catalysing reactions that would not occur under physiological conditions. The presence of metal ions is crucial for the oxidation/reduction processes, electron transfer, spin-forbidden reactions and ‘difficult reactions’, such as N2, O2, C–H bond cleavage (or formation). These processes are intimately involved in the fundamental elements of life, e.g. respiration and photosynthesis. Enormous efforts, both experimental and theoretical, have been exerted to understand the structure and function of metalloproteins. While experiments (e.g., X-ray crystallography, various spectroscopic techniques, electrochemistry) are crucial in initial phases of our understanding to a particular system, theoretical calculations complement these data by providing a unique one-to-one structure-energy mapping.[1] On an example of a multi-copper oxidase,[2-5] and non-heme diiron D9-desaturase,[6,7] I will demonstrate that by correlating experimental and theoretical data, the reaction mechanisms of bioinorganic systems can be fully elucidated. This may eventually lead to a formulation of powerful and qualitative concepts governing catalytic processes involving hydrogen-atom transfer reactions.[8]
|
|
|
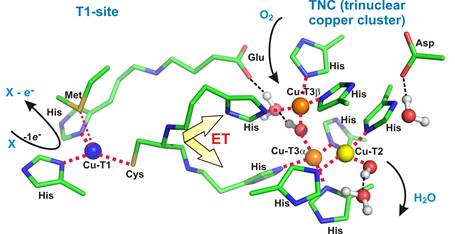
Figure 1. Active Site(s) of Multi-Copper Oxidases. |
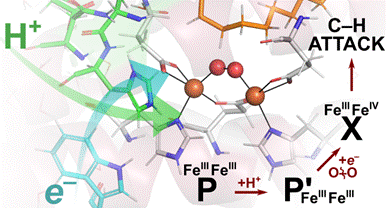
Figure 2. Catalytic Cycle of D9-desaturase. |
1. Rokob, T. A.; Srnec, M.; Rulíšek, L. Dalton Trans. 2012, 41, 5754‑5768
2. Rulíšek, L.; Solomon, E. I.; Ryde, U. Inorg. Chem. 2005, 44, 5612‑5628
3. Ryde, U.; Hsiao, Y.-W.; Rulíšek, L., Solomon, E. I. J. Am. Chem. Soc. 2007, 129, 726‑727
4. Rulíšek, L.*; Ryde, U. Coord. Chem. Rev. 2013, 257, 445‑458
5. Li, J.–L.; Farrokhnia, M.; Rulíšek, L.; Ryde, U. J. Phys. Chem. B 2015, 119, 8268–8284
6. Chalupský, J.; Rokob, T. A.; Kurashige, Y.; Yanai, T.; Solomon, E. I.; Rulíšek, L.; Srnec, M. J. Am. Chem. Soc. 2014, 136, 15977‑15991
7. Bím, D.; Chalupský, J.; Culka, M.; Solomon, E. I.; Rulíšek, L.; Srnec, M. J. Am. Chem. Soc. 2020, 142, 10412−10423
8. Bím, D.; Maldonado-Domínguez, M.; Rulíšek, L.; Srnec, M. Proc. Natl. Acad. Sci. 2018, 115, E10287-E10294