Structural changes of carotenoid echinenone in Orange Carotenoid Protein studied by femtosecond Raman spectroscopy
M. Kloz1, P. Čubáková1, T. Friedrich2 , T.Polivka3, E. Maksimov4
1ELI-Beamlines, Institute of Physics, Praha, Czech Republic.
2Technische Universität Berlin, Institute of Chemistry PC14, Berlin, Germany.
3Institute of Physics, Faculty of Science, University of South Bohemia, České Budějovice, Czech Republic.
3Faculty of Biology, M.V. Lomonosov Moscow State University, Moscow, Russia.
miroslav.kloz@eli-beams.eu
The orange carotenoid protein (OCP) [1] shown in the figure 1 is a perfect system to study changes in cofactor structure during photoswitching of proteins by Raman techniques. It hosts a single xanthophyll molecule and undergoes well-studied (but not yet fully understood) photocycle that is associated with the loss of vibrational structure in the absorption spectra.
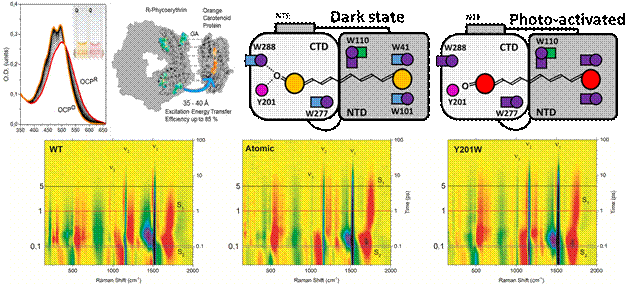
Figure 1
The orange carotenoid protein consists of two subunits that get mutually loose after carotenoid excitation that switches it between the so-called “red” and “orange” states. In that form, it binds to other light-harvesting proteins while greatly increasing their non-radiative decay of excitons. Both the mechanism of OCP photoswitching and its subsequent role as a trigger of non-photochemical quenching is yet to be understood. We studied the wild type and two types of mutants (including utilization of non-canonical amino acids) to understand the role of hydrogen bond formation in the photoswitching mechanism by Stimulated Raman scattering. (figure is with courtesy of Eugen Maksimov)
|
|
|
|
|
|