Mechanisms of Charge Transfer through Multiheme Protein Junctions, Their Distance and Band-Alignment Dependencies
Z. Futera1, X., Wu2, J. Blumberger2
1Faculty of Science,
University of South Bohemia, Branišovská 1760,
370 05 České Budějovice, Czech Republic
2University College
London, Department of Physics and Astronomy,
Gower Street, London WC1E 6BT, United Kingdom
zfutera@prf.jcu.cz
Multiheme cytochromes are redox-active proteins that can efficiently transfer electrons over biological membranes. Electrons flow through such proteins by sequential, thermally activated hops between the heme cofactors and their iron cations, following the Marcus theory of electron transfer. However, experimental measurements on the cytochrome-based junctions between metal contacts in vacuum yielded currents of relatively high magnitudes but practically no temperature dependence [1]. These observations suggested that electrons could coherently tunnel through the protein structures on their way from one electrode to another [2]. Yet, the efficiency of the tunneling mechanism is known to decay exponentially with the distance and cannot explain transport through extraordinary wide junctions.
In the presented theoretical study of stacked small-tetraheme cytochrome (STC) junctions between the gold electrodes, we investigate the distance and band-alignment dependencies of these two mechanisms using DFT calculations and current-voltage curve modeling. We show that the significant potential drop on the protein/metal interface severely hinders the incoherent hopping and supports the off-resonant tunneling as the transport mechanism. However, these two fundamentally different mechanisms begin to be competitive, or their preference is even swapped, as the protein states are brought closer to the electrode Fermi level. Therefore, the specific design of the protein/metal contacts or application of a gate potential controlling the protein electronic levels could drastically affect how electronic charges pass through the redox proteins in nanoelectronic devices.
 (a)
(a) 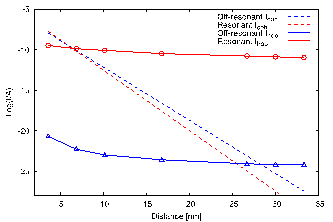 (b)
(b)
Figure 1. (a) DFT model of STC junction between gold electrodes. (b) Predicted current magnitudes using incoherent hopping and coherent tunneling models under resonant and off-resonant conditions.
[1] Garg, K. et al.: Direct Evidence for Heme-Assisted Solid-State Electronic Conduction in Multi-Heme c-Type Cytochromes. Chem. Sci. 9, 7304-7310, 2018.
[2] Futera, Z. et al.: Coherent Electron Transport across a 3 nm Bioelectronic Junction Made of Multi-Heme Proteins. J. Phys. Chem. Lett. 11, 9766-9774, 2020.