Structural Changes and Their Consequences for Azurin
Oxidation in Vacuum and on Gold Interfaces
O. V.
Kontkanen1, D. Biriukov1,2, Z.
Futera1
1Faculty of Science, University of South Bohemia, Branišovská 1760,
370 05 České Budějovice, Czech Republic
2Institute of Organic Chemistry and Biochemistry of the Czech Academy of
Sciences,
Flemingovo náměstí 542, 160 00 Prague 6, Czech Republic
okontkanen@prf.jcu.cz
Charge transfer processes facilitated by metalloproteins play an essential role in biology and technological applications, including novel vacuum-based nanobioelectronic devices. Detail understanding of the charge-transport mechanism and its dependence on the environment of a protein is thus very desirable. In an aqueous solution, electrons flow through biomatter by sequential, thermally-activated hopping between the available redox sites. However, the interaction of the proteins with metal surfaces has profound consequences on the electronic structure, as suggested by the temperature-independent current-voltage response measured in vacuum junctions [1]. Such behavior could be, in principle, explained either by coherent tunneling mechanism or by substantial lowering of the so-called reorganization free energy controlling the barrier heights in the hopping mechanism.
We investigated such environmental effects on structure and oxidation reorganization free energies of the Azurin protein, as extracted to vacuum and consequently adsorbed to clean gold surfaces. For that, we employed molecular dynamics (MD) computational techniques and QM/MM sampling [3] within the framework of density-functional theory (DFT). We reproduced the experimental value of the reorganization free energy in the solution (~0.7 eV). However, the energy is not reduced upon the extraction of Azurin to vacuum due to its increased flexibility near the redox Cu site (c.f. Fig. 1). On the gold surfaces, the reorganization energy varies between 0.6 and 0.9 eV, depending on the particular adsorption structure. The structural flexibility is balanced there with the metal adsorption and polarization effects. In either case, the reorganization free energy is kept relatively high, which does not support the hopping mechanism of electron transport on vacuum biometallic interfaces.
a) 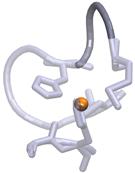 b)
b)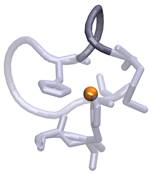 c)
c)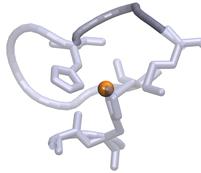
Figure
1. Observed loop twists between His117 and Met121 (dark
grey) next to the redox Cu site (orange).