Crystal structure of heme-based oxygen sensor AfGcHK in complex with imidazole
T. Skalova1, A. Lengalova2, J. Dohnalek1, K. Harlos3, P. Mihalcin2, P. Kolenko1,4, M. Stranava2, J. Blaha2, T. Shimizu2 and M. Martínková2
1Institute of Biotechnology of the Czech Academy of Sciences, v.v.i., Biocev, Vestec, 252 50 Czech Republic
2Department of Biochemistry, Faculty of Science, Charles University, Prague 2, 128 43 Czech Republic
3Division of Structural Biology, The Wellcome Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, OX3 7BN Oxford, United Kingdom
4FNSPE, Czech Technical University in Prague, Břehová 7, Prague 1, 115 19 Czech Republic
t.skalova@gmail.com
AfGcHK [1] is a globin coupled histidine kinase from a soil bacterium Anaeromyxobacter sp. Fw109-5. It is an intracellular enzyme which serves as an oxygen sensor. It has two domains: the N-terminal domain contains heme with an oxygen binding site and the C-terminal domain exhibits autophosphorylation activity induced by the oxygen binding. Both domains are connected by a flexible linker, which makes crystallization of the full-length AfGcHK difficult. The enzyme is active in dimeric form.
Two crystal structures of the globin domain of the enzyme, comparing its oxidized and reduced state [2], were presented in this conference two years ago. This year, we would like to present crystal structure of the AfGcHK globin domain in a monomeric form, in complex with imidazole [3].
The crystal grew in the Morpheus C4 condition containing 50 mM imidazole as a part of a buffer system. The diffraction data were collected at the Diamond Light Source and they were processed to resolution 1.8 Å. The structure has been deposited in the Protein Data Bank under the code 6OTD.
The AfGcHK globin domain has monomeric form in this crystal structure. Imidazole is bound to the heme oxygen binding site and, moreover, there is a second bound imidazole molecule. It is also near the heme and occupies position of Tyr15 of the partner chain in dimer. This observation led us to study the importance of the Tyr15 residue for the globin domain dimerization and enzyme activity.
We discovered that mutation of Tyr15 is a simple way to disrupt the dimerization interface of the AfGcHK globin domain. It was found that the dimerization of the globin domain is necessary for internal signal transduction in the full-length AfGcHK and for its autophosphorylation activity (Fig. 1).
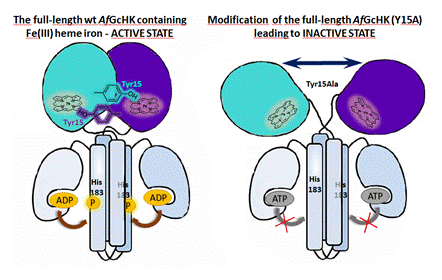
Figure 1. Scheme of two-domain dimeric histidine kinase AfGcHK (left). When globin domain dimerization is disrupted, the enzyme loses its activity (right). This figure was originally published in the Journal of Biological Chemistry [3]. © the American Society for Biochemistry and Molecular Biology
1. M. Martínková, K. Kitanishi, T. Shimizu, J. Biol. Chem., 288, (2013), 27702–27711.
2. M. Stranava, P. Man, T. Skálová, P. Kolenko, J. Blaha, V. Fojtikova, V. Martínek, J. Dohnálek, A. Lengalova, M. Rosůlek, T. Shimizu, M. Martínková, J. Biol. Chem., 292, (2017), 20921–20935.
3. T. Skalova, A. Lengalova, J. Dohnalek, K. Harlos, P. Mihalcin, P. Kolenko, M. Stranava, J. Blaha, T. Shimizu, M. Martínková, J. Biol. Chem., 295(6), (2020), 1587–1597.
This work was supported in part by the Grant Agency of Charles University in Prague (grant 704217), by Charles University (SVV260427/2018), by the ERDF fund (projects CZ.1.05/1.1.00/02.0109, CZ.02.1.01/0.0/0.0/16_013/0001776, and CZ.02.1.01/0.0/0.0/15_003/0000447), and in part by the MEYS CR (grant CZ.02.1.01/0.0/0.0/16_019/0000778).