Structural and biophysical study of the first N-terminal insert of tau protein in the complex with mononclonal antibody
I. Jahodova1,2, E. Janisikova3, A. Legenova3, R. Skrabana2,3
1 Department of Biochemistry, Faculty of Natural Sciences, Comenius University in Bratislava, Mlynska dolina, Ilkovicova 6, 842 15 Bratislava
2 Institute of Neuroimmunology, Slovak Academy of Sciences, Dubravska cesta 9, 845 02 Bratislava
3Axon Neuroscience R&D Services SE, Dvorakovo Nabrezie 10, 811 02 Bratislava
jahodovaiveta@centrum.sk
Alzheimer's disease (AD) is neurodegenerative disorder characterized by deposits of β-amyloid plaques and aggregated tau protein. Tau is associated with microtubules and stabilizes them maintaining their highly dynamic state. Under disease conditions tau undergoes aberrant posttranslational modifications, e.g. truncation and hyperphosphorylation [1]. Pathological form of tau protein does not bind microtubules and is prone to aggregation creating dimers and oligomers, which next are forming straight filaments and paired helical filaments leading to typical neurofibrillary tangles.
Tau protein is an intrinsically disordered protein (IDP) without any stable secondary or tertiary structure [2]. The conformational regulation and structural changes of tau are of great interest because they can be targeted in the treatment of AD. For monitoring the structure, dynamic biophysical methods are employed. Very useful can be studies involving interaction partners of tau, particularly monoclonal antibodies recognizing specific (conformational) epitopes on tau. Biophysical characterization of tau-antibody complexes may elucidate kinetic and thermodynamic regulation of individual tau epitopes, whereas crystallography of complexes may confer molecular details of tau structural propensity.
Monoclonal antibody DC39N1 used in this work was prepared after mice immunization with short peptide corresponding to the first alternatively spliced N-terminal insert of tau. It was used for detailed analysis of the structure of N-terminal domain of tau protein alone and in cooperation with other tau protein domains. Previously, DC39N1 epitope was refined to nine amino acids from 58 to 67 residues in 2N4R isoform [3, 4].
Fab of DC39N1 was cloned in eukaryotic expression vector and produced in ExpiCHO cells. After cultivation, the antibody was purified by affinity chromatography, characterised by dynamic light scattering and SDS-PAGE and prepared for isothermal titration calorimetry (ITC), surface plasmon resonance (SPR) and crystallisation experiments. ITC and SPR measurements of complex formation with a large panel of tau proteins revealed subtle allosteric regulation of flexible tau protein by alternatively spliced parts of molecule. In crystallization experiments we were able to obtain crystals of antibody alone and of putative complexes with four tau protein variants of sufficient quality (Fig. 1).
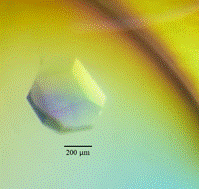
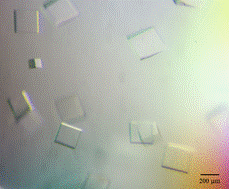
Figure 1. Microphotography of crystals created by Fab monoclonal antibody DC39N1 (left) and the DC39N1 Fab in complex with tauN98 (right). Crystal of Fab monoclonal antibody DC39N1 grown in 0.1M Sodium HEPES pH7.5 10%w/v PEG 6000 5%v/v MPD and crystal of the DC39N1 Fab in complex with tauN98 grown in 0.2 M Ammonium sulfate, 0.1 M BIS-TRIS pH 6.5, 25% w/v PEG 3350.