Preparation of a set of anti-tau monoclonal antibodies in CHO cell line using a robust system for eukaryotic expression
K. Heskova1,2, L. Hornakova1,2, I. Jahodova1,2, K. Meskova2, R. Skrabana2,3, A. Idunkova3, E. Janisikova3,
1 Department of Biochemistry, Faculty of Natural Sciences, Comenius University in Bratislava, Mlynska dolina, Ilkovicova 6, 842 15 Bratislava
2Institute of Neuroimmunology, Slovak Academy of Sciences, Dubravska cesta 9, 845 02 Bratislava
3 AXON Neuroscience R&D Services SE, Dvorakove Nabrezie 10, 811 02 Bratislava
heskovakatarina@gmail.com
Alzheimer's disease (AD) is characterized by dementia, which usually begins with subtle changes and memory loss and slowly increases in severity until it affects the patient's daily life and eventually ends in death of the patient. AD is pathologically defined by deposits of aggregated β-amyloid (Aβ) and hyperphosphorylated tau protein. In pathological conditions, tau polymerizes to neurofibrillary tangles composed of tau filaments, which overlap with regions of neuronal loss in AD and related tauopathies. Monoclonal antibodies are in many instances sole tools able to reveal subtle conformation changes on tau leading to neurodegeneration. A method permitting reproducible preparation of monoclonal antibodies is highly needed for structural, biophysical and functional studies on tau, with the consequences for the development of diagnostics and therapy of AD.
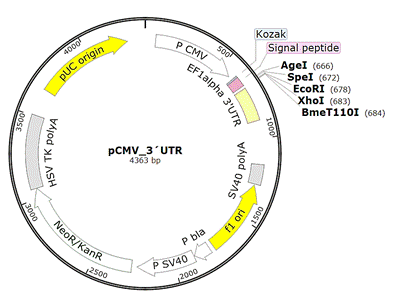
We constructed a eukaryotic expression vector pCMV_3'UTR derived from the pCMV-Script backbone. The new vector contains CMV promoter, signal peptide driving expression to culture media, multiple endonuclease cleavage sites for flexible cloning and 3´ untranslated region of EF1 alpha gene, which starts immediately after the sequence of antibody chain (Fig.1). The presence of 3'UTR domain enhances the level of expression in CHO cell line.
We have analysed the production of recombinant form of Fab monoclonal antibodies DC25, MN423, DC11 and DC39N1 cloned in the pCMV_3'UTR vector. The antibodies were purified by affinity chromatography on Protein G. We compared three different protocols for CHO cell cultivation after transfection. In the final optimized conditions, we were able to prepare more than 10 mg of purified antibody from 40 ml culture in three weeks. Prepared Fabs were successfully used for crystallization of tau protein complexes and biophysical studies.
 A
A
B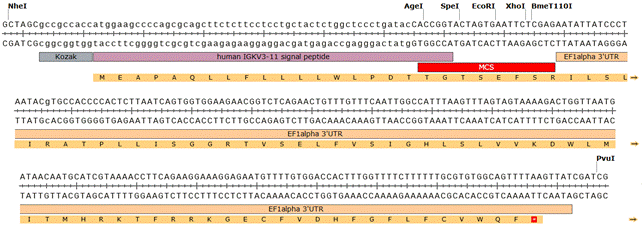
Figure 1. Overview of new vector. A - Map of pCMV_3'UTR vector with highlighted sequence features (P- promoter). AgeI and XhoI were chosen as cloning sites for monoclonal antibodies used in this study. B – Detailed view of pCMV_3'UTR vector with highlighted endonuclease sites, human IGKV3-11 signal peptide and elongating factor 1 alpha 3´UTR.