Study of DNA-Protein Interactions in Living Cells by in-cell NMR Spectroscopy
Michaela Krafčíková1,2,*, Letizia Barbieri3, Enrico Luchinat3, Martina Lenarčič Živković4,5, Radovan Fiala4, Silvie Foldynová-Trantírková1,4, Lukáš Trantírek4
1 Institute of Biophysics of the AS CR, v.v.i., Královopolská 135, 612
65, Brno, Czech Republic
2NCBR, Faculty of
Science, Masaryk University, Kamenice 753/5, 625 00, Brno, Czech Republic
3 CERM, University of Florence, Via Luigi Sacconi 6, 50019 Sesto Fiorentino, Italy
4 Central European Institute of Technology, Masaryk University, Kamenice 753/5, 625 00, Brno, CZ
5Slovenian NMR Centre, National Institute of Chemistry, Hajdrihova 19, SI-1000 Ljubljana, Slovenia
* krafcikova@ibp.cz
In each organism, there are macromolecules - elementary components of living machinery responsible for the proper function of their cells. There are number of established structural and chemical biology approaches to investigate relationships between macromolecule structure and function under in vitro conditions. However, conventional structural and chemical biology methods for analysis of macromolecules are not conducted in their native context. Consequently, information on important structural and functional features of macromolecules are lost as macromolecules inside the living cells are influenced by many environmental factors such as temperature, molecular crowding, pH, viscosity, or metabolites.
In-cell NMR spectroscopy can provide invaluable biological, structural, and functional information about behavior of proteins and nucleic acids (DNA/RNA) in the complex physiologically relevant environment of living cells at close-to-atomic resolution1. Here, we report on development of the in-cell NMR based approach for investigation of DNA-protein interactions in living human cells (Fig.1). The procedure is based on inducible overexpression of the isotopically labeled protein of interest encoded by corresponding DNA sequence stably incorporated in human genome of cells that are subsequently transfected with desired DNA/RNA target. Our ambition is to reconstitute protein-NA binding interface susceptible to in-cell NMR investigation inside intracellular space of living cells.
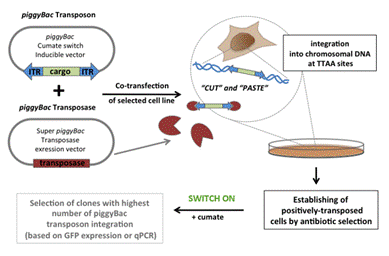
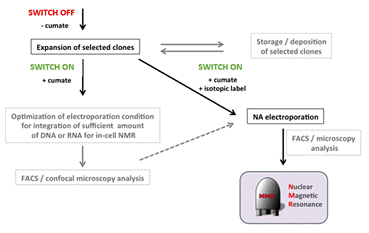
Figure 1. Scheme of DNA-protein experiment.
1. Luchinat E, Banci L. A Unique Tool for
Cellular Structural Biology: In-cell NMR. J Biol Chem. 2016 Feb
19;291(8):3776–84
Acknowledgement: This research was supported by the grant from Czech Science Foundation (19-26041X), by the grant from Ministry of Health of the Czech Republic (NV19-08-00450), by the project MSCAfellow2@MUNI (CZ.02.2.69/0.0/0.0/18_070/0009846) by Ministry of Education, Youth, and Sports (MEYS) of the Czech Republic, and by the project SYMBIT (CZ.02.1.01/0.0/0.0/15_003/0000477) funded by the European Regional Development MEYS of the Czech Republic. MEYS is also acknowledged for its support of access to research infrastructure (CEITEC 2020 LQ1601; CIISB-LM2018127; Czech-BioImaging LM2018129; EATRIS-CZ LM2018133).