Comparative ultrafast spectroscopy and structural analysis of OCP1 and OCP2 protein from Tolypothrix
Valentyna Kuznetsova1, Maria Agustina
Dominguez-Martin2, Han Bao2, Sayan Gupta3,
Markus Sutter2,3,4, Miroslav Kloz5, Mateusz Rebarz5,
Martin Přeček5, Yan Chen4, Christopher J. Petzold4,
Corie Y. Ralston3, Cheryl A. Kerfeld2,3,4,6, Tomáš Polívka1
1Institute of Physics, Faculty of Science, University of South Bohemia, Branišovská 1760, 370 05 České Budějovice, Czech Republic
2MSU-DOE Plant Research Laboratory, Michigan State University, East Lansing, MI 48824, USA
3Molecular Biophysics and Integrated Bioimaging Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
4Environmental Genomics and Systems Biology Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
5ELI Beamlines, Institute of Physics, Czech Academy of Sciences, Za Radnicí 835, 252 41 Dolní Břežany, Czech Republic
6 epartment of Biochemistry and Molecular Biology, Michigan State University, East Lansing, MI 48824, USA
The orange carotenoid protein (OCP) is a structurally and functionally modular photoactive protein involved in cyanobacterial photoprotection. Recently, based on bioinformatic analysis and phylogenetic relationships, new families of OCP have been described, OCP2 and OCPx. The first characterization of the OCP2 showed both faster photoconversion and back-conversion, and lower fluorescence quenching of phycobilisomes relative to the well characterized OCP1. Moreover, OCP2 is not regulated by the fluorescence recovery protein (FRP). In this work, we present a comprehensive study combining ultrafast spectroscopy and structural analysis to compare the photoactivation mechanisms of OCP1 and OCP2 from Tolypothrix PCC 7601. We show that despite significant differences in their functional characteristics, the spectroscopic properties of OCP1 and OCP2 are comparable. This indicates that the OCP functionality is not directly related to the spectroscopic properties of the bound carotenoid. In addition, the structural analysis by X-ray footprinting reveals that, overall, OCP1 and OCP2 have grossly the same photoactivation mechanism. However, the OCP2 is less reactive to radiolytic labeling, suggesting that the protein is less flexible than OCP1. This observation could explain fast photoconversion of OCP2.
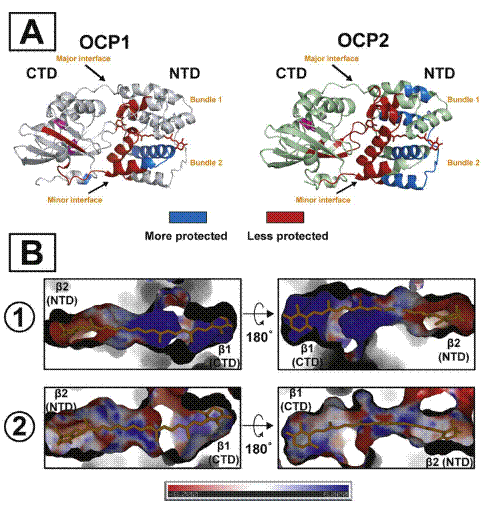
Figure 1. A. Local structural changes in the Tolypothrix OCP1 and OCP2 upon light activation as measured by XFMS. The changes in solvent accessibility (> 1.5-fold changed) upon light activation are plotted on the OCP1 crystal structure (PDB 6PQ1) and on the OCP2 homology model showing regions becoming less protected (red) and more protected (blue). The carotenoid in OCPR form is shown in red sticks. The domain separation is not shown. The conserved Y201 and W288 are highlighted in magenta. B. Charge distribution of the carotenoid tunnel in OCP1 (1) and OCP2 (2). β1 and β2 regions are highlighted, colored by electrostatic potential from –6kT/e (red) to 6kT/e (blue).