Vibrationally-resolved UV-absorption and magnetic circular dichroism of nucleosides
T. Karthick, J. Kaminský, P. Bouř
Institute of Organic Chemistry and Biochemistry, Academy of Sciences, Flemingovo namestí 2, 16610 Prague, Czech Republic
karthick.thangavel@uochb.cas.cz
Nucleobase pairing enables the encoding of genetic information in DNA and RNA. Thus far, the interaction of (deoxy)ribose with the nucleobases has been hard to explain from optical spectra, which is compounded by the lack of understanding of sugar conformations. Circular dichroism (CD) spectra provide clear understanding of molecular conformations. Despite of numerous experimental and theoretical CD studies on the base components of nucleic acids reported earlier [1-3], transitions in the UV-vis region that are too weak to be seen in normal CD require further elucidation. For example, the experimental absorption spectrum of adenosine (Figure 1) shows a strong absorption apparently broad at 259.2 nm. The recent studies on the adenine nucleobase [1] and deoxyadenosine [4] reported the band at 260 and 259.7 nm, respectively. This implies that the effect of sugars on the absorption spectrum of adenine seems to be insignificant.
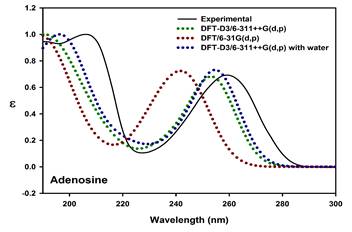
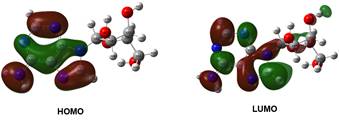
Figure 1. UV absorption spectrum (Top) and frontier molecular orbitals (Bottom) of adenosine
Magnetic circular dichroism (MCD) is a useful tool for precise analysis of the electronic states of a molecule, and it resolves bands that are not obvious from absorption spectrum. The present work attempts to investigate the magnetically induced chirality of electronic transitions of the nucleosides, and to assign spectral bands more precisely. The effect of the sugar components on the electronic transition of nucleobases is interpreted. The possible conformations along the β-glycosidic bond were evaluated using DFT theory. TDDFT calculation and MCD simulation results were used to interpret the experimental spectra. Simulation of MCD spectra is obtained from TDDFT using sum over state (SOS) summation [5,6]. SOS involves a quadruple sum over the virtual and occupied molecular orbitals [6]. In general, MCD intensities are described by Faraday terms A, B and C in which origin-independent expression (localized orbital/localized origin) of the MCD tensor is used to obtain the B term. In addition, vibrationally-resolved absorption and MCD spectra of nucleosides such as adenosine and methyluridine were generated in order to consider the dynamic effects during the electronic transition. The band shape of the prominent absorption of adenosine corresponding to HOMO®LUMO transition is reproduced by theoretical vibronic spectrum (Figure 2). For simple systems, the experimental and simulated MCD spectra were nearly matching.
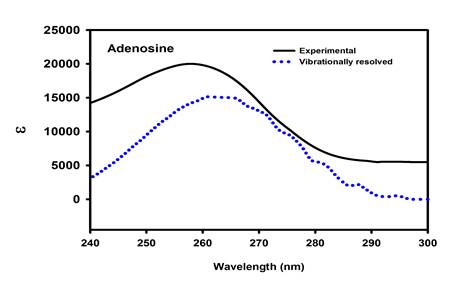
Figure 2. Vibrationally-resolved absorption of adenosine
In Figure 3, the absorption, CD and MCD spectrum of methyluridine is presented. Methyluridine produces two broad bands; one at 267.2 nm responsible for two electronic transitions (H®L and H-1®L) and another at 205.9 nm. It is obvious from the theoretical UV (Figure S1, Supplementary Material) and CD spectrum of methyluridine, the absorption around 200 nm in the UV spectrum is a composite and it corresponds to the peaks having small rotatory strengths near 205 and 194 nm in CD spectrum. MCD spectrum shows the magnetically enhanced CD bands in opposite orientations of the samples with respect to the magnetic poles. The simulated MCD reproduces experimental MCD spectrum. However, over all blue shift is observed in most of the cases.
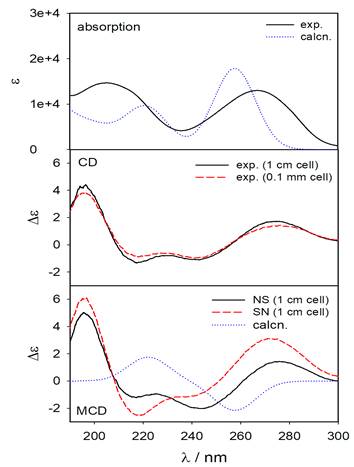
Figure 3. Absorption, CD and MCD spectrum of methyluridine
The author Karthick Thangavel acknowledges the Institute of Organic Chemistry and Biochemistry for Internal Postdoctoral Fellowship Program and large part of the computations was performed at the National Grid Infrastructure (Metacentrum, CESNET program LM2015042 and the CERIT Scientific Cloud LM2015085).