Structural and functional analysis of the ACBD3-3A complexes in enterovirus infection
Vladimira Horova1, Heyrhyoung Lyoo2,
Dominika Chalupska1, Miroslav Smola1, Jana Humpolickova1,
Jeroen RPM Strating2, Frank JM van Kuppeveld2,
Evzen Boura1, and Martin Klima1
1Institute of Organic Chemistry and Biochemistry, Czech Academy of Sciences, Prague, Czech Republic
2Faculty of Veterinary Medicine, Utrecht University, Utrecht, The Netherlands
vladimira.horova@uochb.cas.cz
Enteroviruses, members of the family Picornaviridae, are small single stranded RNA viruses with positive-strand polarity and non-enveloped icosahedral capsids. Depending on species enteroviral infections lead either to the asymptomatic or mild respiratory diseases, but also cause severe illnesses as acute hemorrhagic conjunctivitis, meningitis, myocarditis, encephalitis, or poliomyelitis [1].
Viral infection affects a lot of cellular processes and enteroviruses need host protein machinery for their successful replication. ACBD3 was described to be in different cases of picornaviruses a crucial player in viral RNA replication due to its influence on reorganization of intracellular membranes. The C-terminal GOLD domain of ACBD3 has been reported to interact with the golgin B1, which results in the Golgi localization of ACBD3 [2]. However, in enterovirus-infected cells, the ACBD3 GOLD domain interacts preferentially with viral non-structural 3A proteins, which causes re-localization of ACBD3 to the sites of virus replication [3]. The structural determinants of its recruitment to the viral replication sites are poorly understood.
Here we describe structures of GOLD:3A complexes from four representative enteroviral species. Using mutation analysis we identified amino acid residues important for the ACBD3:3A interaction, co-localization, stimulation of PI4KB recruitment, and facilitation of virus replication in human cells. Interestingly the enterovirus and previously described kobuvirus 3A proteins bind to the same regions of the ACBD3 GOLD domain but in opposite orientation.
We found that ACBD3: enterovirus 3A complexes form heterotetramers consisting of two molecules of the viral 3A protein and two molecules of host ACBD3. We identified also that LVVY 3A mutants remain monomers (Fig. 1).
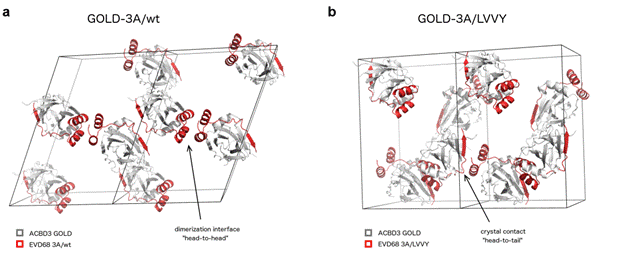
Figure 1. Analysis of the dimerization interface of the GOLD-3A complexes. Crystal packing of the wild-type GOLD -EVD68 3A fusion protein (a) and its LVVY mutant (b).
In conclusion, in our study we analysed in detail molecular interactions between entreroviral 3A protein and human ACBD3 GOLD domain and we showed a conserved mechanism how diverse enterovirus species recruit the ACBD3 protein. In comparison with kobuvirus 3A we also found nice example of convergence in picornavirus evolution.
1. Tapparel, C., Siegrist, F., Petty, T.J. & Kaiser, L., Infect Genet Evol 14, (2013), 282-93.
2. Sohda, M. et al., J Biol Chem 276, (2001), 45298-45306.
3. Lei, X. et al., Sci Rep 7, (2017), 44592.