Structure of Leishmania RNA virus 1
M. Procházková1, T. Füzik1, D. Grybtchuk1,
L. Podešvová2, F. Falginella1,
R. Vácha1, V. Yurchenko2, P.
Plevka1
1CEITEC-MU, Kamenice 753/5, 625 00 Brno, CZ
2LSRC, Faculty of Science, University of Ostrava, Chitussiho 10, 710 00 Ostrava, CZ
michaela.veselikova@ceitec.muni.cz
Leishmania RNA virus 1 (LRV1, Totiviridae) infects human protozoan parasite Leishmania. LRV1-carrying parasites are more virulent and cause more serious mucocutaneous form of leishmaniasis characteristic by massive inflammation and facial tissue damage [1,2]. Despite its role in pathogenesis of leishmaniasis, the LRV1 was not structurally characterized up to date. Here we present a structure of LRV1 capsid and demonstrate that it binds the host RNA.
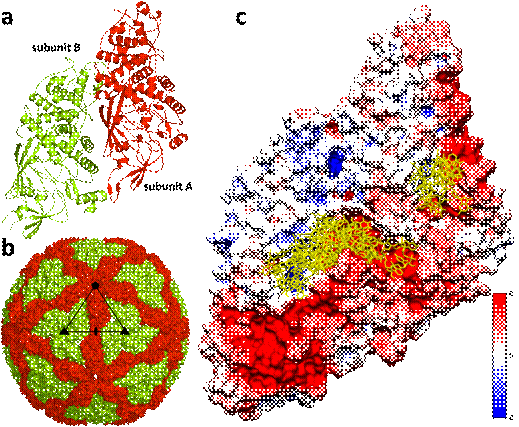
To circumvent the issue of low virus titer in Leishmania cells, we took advantage of the recombinant capsid protein tendency to form stable virus-like particles (VLPs). The LRV1 VLP is an empty particle 42 nm in diameter with an icosahedral symmetry (Fig 1, b) and triangulation number T=*2 typical for totiviruses. The asymmetric unit consists of two copies of the capsid protein (Fig 1, a). We determined the structure of LRV1 VLP to the resolution of 3.65 Å using cryo electron microscopy. The LRV1 capsid protein model was constructed ab initio and refined against experimental data in Phenix suite.
Capsid proteins of related L-A virus of yeast [3] possess the ability to de-cap cellular mRNAs to overload the RNA degradation machinery of the host cell and thus protect viral mRNAs [4,5]. The residues responsible for m7GTP binding and hydrolysis (His154, Tyr150, Asp152, and Tyr452) are not conserved between L-A virus and LRV1. Instead, the molecular simulations point at the cap4 preference for positively charged amino acids on the bottom of a trench formed by three antiparallel alpha-helices in subunit B at the interface with subunit A (Fig 1, c).
Figure 1. (a) Structure of the LRV1 capsid protein asymmetric unit (subunit A in red, subunit B in green). (b) Capsid of LRV1 with icosahedral symmetry axes marked – threefold (triangle), twofold (oval), fivefold (pentagon) and subunit A in red, subunit B in green. (c) Electrostatic surface coloring of LRV1asymmetric unit (red-blue) with top 10 docked cap4 structures in yellow.
1. Hartley M-A, Ronet C, Zangger H, Beverley SM, Fasel N. Frontiers in Cellular and Infection Microbiology. 2012;2: 99.
2. Motta L, Ferreira C, Ito MM, Porrozzi R, Cupolillo E, Godoi R De, et al. PLOS Neglected Tropical Diseases. 2015;9.
3. Naitow H, Tang J, Canady M, Wickner RB, Johnson JE. Nature Structural Biology. 2002;9: 725–728.
4. Blanc A, Ribas JC, Wickner RB, Sonenberg N. Molecular and cellular biology. 1994;14: 2664–74.
5. Masison DC, Blanc A, Ribas JC, Carroll K, Sonenberg N, Wickner RB. Molecular and cellular biology. 1995;15: 2763–71.