Femtosecond vibration spectroscopy of unprotonated retinal in a UV absorbing rhodopsin biological photoreceptor
Miroslav Kloz1,3, Yusaku Hontani1, Matthias Broser2, Meike Luck2, Jörn Weißenborn1, Peter Hegemann2 and John T.M. Kennis1
1Department of Physics and Astronomy, Vrije Universiteit Amsterdam, Amsterdam 1081 HV, De Boelelaan, The Netherlands
2Institut für Biologie, Experimentelle Biophysik, Humboldt Universität zu Berlin, Invalidenstrasse 42, D-10115 Berlin, Germany
3ELI-Beamlines,
Institute of Physics, Na Slovance 2, 182 21 Praha 8, Czech Republic
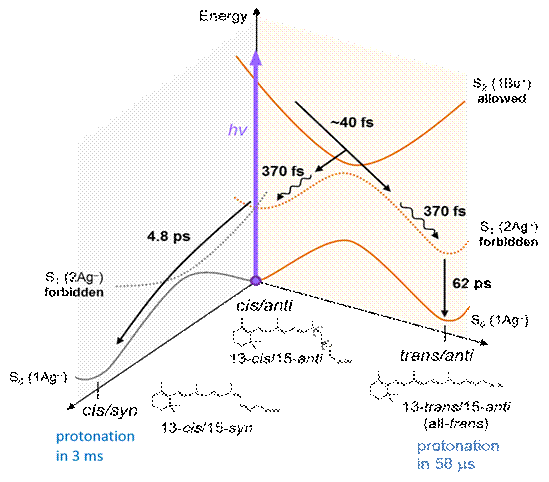
Histidine kinase rhodopsin 1 (HKR1) is a unique UV-absorbing microbial rhodopsin featuring unprotonated retinal Schiff-base. It serves as a model system of UV-absorbing animal rhodopsins, including those of H. sapiens. Unlike the situation for canonical rhodopsins which bind a protonated retinal Schiff base, the photoreaction dynamics of unprotonated retinals have remained essentially unknown. We report the photoisomerization and protonation dynamics of HKR1 probed by transient absorption (TA) and femtosecond stimulated Raman spectroscopy (FSRS) from the femto- to submillisecond timescales. We demonstrate that energy level ordering is inverted with respect to canonical rhodopsins, i.e. that photoexcitation occurs from the S0 to the S2 state, and that the lowest-lying S1 state is optically forbidden. We observe that photoexcitation elicits a double isomerization reaction on distinct potential energy surfaces: optical excitation to the S2 state results in C13=C14 trans-cis isomerization on the S2 - S1 evolution in 40 fs, followed by C15=N16 anti-syn isomerization on the S1 - S0 evolution in 4.8 ps. This results in two deprotonated ground-state photoproducts, all-trans/15-anti and 13-cis/15-syn. Protonation of the former occurs in 58 microseconds, whereas the latter is protonated in ~3 ms, resulting in a stable blue-absorbing form of HKR1 comprising two distinct protonated retinal Schiff base conformers. We thus demonstrate the complete excited-state and ground-state dynamics of protein-bound unprotonated retinal Schiff base, which constitutes a benchmark of the photochemistry of UV-absorbing rhodopsins of all kingdoms of life.
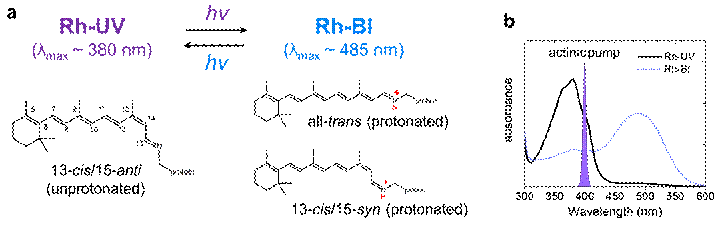
Figure 1. Retinal conformers in HKR1. (a) UV-absorbing (Rh-UV1) and blue-absorbing (Rh-Bl) states of HKR1. (b) Steady-state absorption spectra of Rh-UV1 (black solid line) and Rh-Bl (blue dotted line) states.
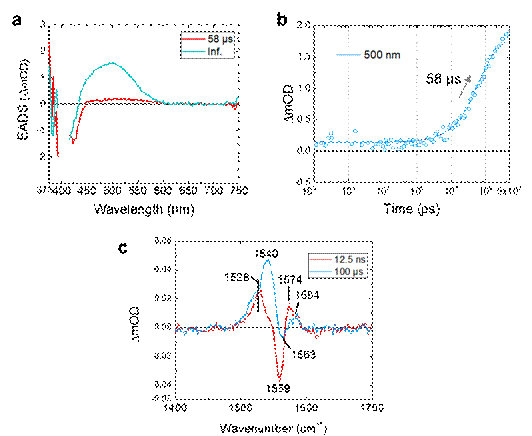
Figure 2. Evolution associated Decay spectra (EADS) of the 58 µs and infinite components of the transient absorption experiments. (a) EADS of the transient absorption signals. (b) A ns-µs time trace at 500 nm of the transient absorption experiments. The open dots and the solid line show raw data and a fitting curve, respectively. (c) FSRS spectra taken at 12.5 ns and 100 ms time delay.