Structural basis of histone deacetylase 8 (HDAC8) selective inhibition
Martin Marek1 and Christophe Romier2
1Loschmidt Laboratories,
Masaryk University, Brno, Czech Republic, Kamenice 5/A13, 62500 Brno, Czech
Republic, email: martin.marek@recetox.muni.cz
2Integrative Structural Biology Programme, IGBMC, Laurent Fries 1,
67400 Illkirch, France
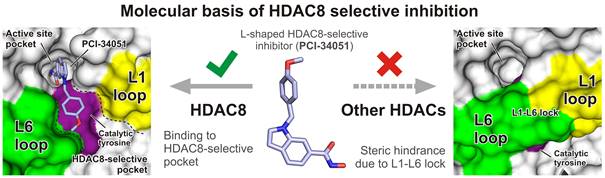
Metal-dependent histone deacetylases (HDACs) are key epigenetic regulators that represent promising therapeutic targets for the treatment of numerous human diseases. Yet, currently FDA-approved HDAC inhibitors non-specifically target most of the eleven structurally similar but functionally different HDAC isozymes, which hampers their broad usage in clinical settings. Selective inhibitors targeting single HDAC isozymes are being developed, for example HDAC8-selective inhibitors PCI-34051 and NCC-149, but precise understanding in molecular terms of their selectivity remains sparse. Here, combination of biochemical, biophysical and structural analyzes of HDAC8 inhibition reveals how selective inhibition is achieved. Our numerous crystallographic structures of HDAC8-inhibitor complexes established with a lattice-free environment at the active site provide unprecedented structural information on the binding of selective inhibitors to HDAC8. Notably, we show that HDAC8-selective inhibitors adopt a characteristic L-shaped conformation, which is required for their binding to a HDAC8-specific pocket formed by HDAC8 active site catalytic tyrosine and HDAC8 L1 and L6 loops. In other HDAC isozymes, presence of a L1-L6 lock sterically prevents L-shaped inhibitor binding. Importantly, shielding of the HDAC8-specific pocket by protein engineering markedly decreases potency of HDAC8-selective inhibitors but also affects catalytic activity. Collectively, our results unravel key HDAC8 active site structural and functional determinants important for catalysis and selective inhibition, which sheds new light on rational design of next-generation chemical probes and epigenetic drugs.
Graphical abstract
Marek M., Shaik T. B., Heimburg T., Chakrabarti A., Lancelot J., Ramos Morales E., Da Veiga C., Kalinin D., Melesina J., Schmidkunz K., Suzuki T., Holl R., Ennifar E., Pierce R. J., Jung M., Sippl W., and Romier C. (2018). Characterization of histone deacetylase 8 (HDAC8) selective inhibition reveals key active site structural and functional determinants. Journal of Medicinal Chemistry 61(22):10000-10016.