Guanine quadruplexes [1] are widely studied
because of their role in various biological processes including gene expression
and cell immortalization, and possible nanotechnological applications2.
G-quadruplexes can be formed under specific physico-chemical conditions from
DNA as well as RNA oligo- or polynucleotides containing repetitive sequences of
several consecutive guanosine residues. DNA quadruplexes exhibit greater
structural polymorphism than RNA, as they can adopt antiparallel, various
hybride (3+1) and parallel folds, whereas RNA quadruplexes seem to be restricted
simply to parallel form. Basic building blocks of G-quadruplex consist of four
guanines interconnected by Hoogsteen hydrogen bonding to form G-quartet,
stabilized by coordinated metal cation, especially Na+ or K+.
G-quartets are stacked one upon the other.
However, G-quartets and their
supramolecular self-associates resembling G-quadruplexes can be formed also
from monomeric guanosine-phosphates. Although G-quartets and self-associates of
5’-rGMP have been thoroughly studied in the presence of various alkali metals by
various methods, notably NMR6, similar studies of deoxyribonucleotide
5’-dGMP are still rare. In particular a direct comparison of the ability of monomeric
5’-rGMPs and 5’-dGMPs to constitute G-quartets and self-associates stabilized
by the most common alkali ions Na+ and K+ is missing,
although it may contribute for better understanding of the reasons of different
polymorphism of DNA and RNA G-quadruplexes in Na+ and K+
solutions.
Here we report results of a
systematic study of the effect of the ribose versus 2’-deoxyribose on
the 5’-GMP self-assembling in pH-neutral or slightly basic Na+ and K+
solutions by means of Raman and 1H and 31P NMR
spectroscopy. Although not yet frequently used in the G-quadruples research,
Raman spectroscopy is especially suitable for the task, since it can be conveniently
used at high 5’-GMP concentrations favorable to self-aggregation. Moreover, Raman
spectra provide well-established spectral markers sensitive to nucleotide
conformation, formation of hydrogen bonds and stacking interactions3,4.
In the present study, the nucleotide concentration, nature, concentration and
stochiometry of alkali cations, and temperature were systematically varied to
find that ability of 5’-dGMP to constitute G-quartets and ordered supramolecular
structures stabilized by Na+ is substantially lower than that of
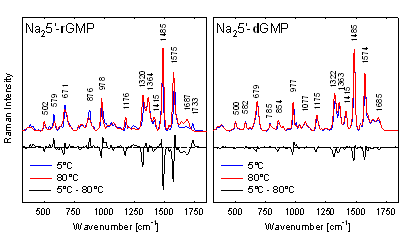
5’-rGMP. As shown on Fig. 1, 5’-dGMP remained in Na+ solution as
monomer even at 800 mM concentration where Na25’-rGMP clearly form
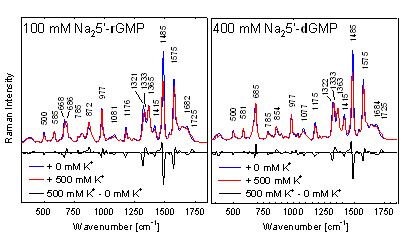
firmly stacked G-quartets. On the other hand, after introduction of K+
ions both guanosine-monophosphates readily form self-stacking G-quartets,
although self-association of 5’-rGMP was still greater than that of 5’-dGMP
(Fig. 2). Possible consequences for G-quadruplex polymorphism will be
discussed.