Second generation of Carborane-Based Inhibitors of Carbonic Anhydrases
Jiří Brynda1,2, Petr Pachl1,2, Václav Šícha3, Milan Fábry1, Bohumír Grüner3, Petr Cígler2, Pavlína Řezáčová1,2
1Institute of Molecular Genetics Academy of Sciences of the Czech Republic, v.v.i.
2Institute of Organic Chemistry and Biochemistry AS CR, v.v.i.
3Institute of Inorganic Chemistry, Academy of Sciences of the Czech Republic, v.v.i.
Carbonic anhydrases (CAs) play important roles in many physiological and pathophysiological processes. For example, extracellular CAs participate in tumor growth and progression . CAIX, which is selectively expressed in a range of hypoxic tumors, is a validated diagnostic and therapeutic target (recently reviewed in [1–3]). There are 15 human CA isoenzymes, and due to the ubiquity of these enzymes in human tissues, selective inhibition is a very important aspect of drug design.
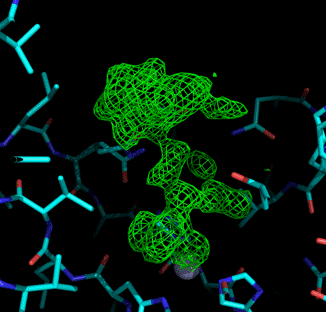
Mechanism of the inhibition was elucidated for paternal compounds by solving crystal structure of the CA-IX like in complex with these inhibitors. The CA-IX-like enzyme has the active site analogous to CA-IX thanking to site directed mutagenesis of CAII and thus behaves as CAII, it means that crystallizes very well. These x-ray structures proved specific binding of the cluster compounds in the active site of CA-IX and they revealed the key interactions, which are responsible for binding and inhibition at molecular level. Crystal structures were solved for CA-IX-like enzyme in complex with all essential types of carborane and (metalla)carborane inhibitors with both, sulphamide and sulphonamide functional group, several of them with atomic resolution. The structural information has been is consecutively exploited for design of structurally optimized generations of inhibitors.
The study is supported by the Czech Science Foundation (GA CR) grant no. 15-05677S.
1. Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD. The role of carbonic anhydrase 9 in regulating extracellular and intracellular pH in three-dimensional tumor cell growths. The Journal of Biological Chemistry. 2009;284(30):20299–20310.
2. McDonald PC, Winum J, Supuran CT, Dedhar S. Recent developments in targeting carbonic anhydrase IX for cancer therapeutics. Oncotarget. 2012;3(1):84–97.
3. Lock FE, McDonald PC, Lou Y, et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene. 2013;32(44):5210–5219.