Phase transition approach on the interpretation of the chemical oscillation in the Belousov-Zhabotinsky reaction
Anna Zhyrova, Dalibor Štys, Renata Rychtáriková and Tomáš Náhlík
University of South Bohemia in Ceske Budejovice, Faculty
of Fisheries and Protection of Waters, South Bohemian Research Centre of
Aquaculture and Biodiversity of Hydrocenoses, Institute of Complex Systems, Zámek
136, 373 33 Nové Hrady, Czech Republic
zhyrova@frov.jcu.cz
Actively studied over the last decades the Belousov-Zhabotinsky (BZ) reaction [1] is no lost interest in the scientific world nowadays. The BZ reaction was devised as a primitive model of citric acid cycle [2]. To the surprise of the authors, it brought about the phenomenon of chemical clock (in mixed systems) or spontaneous pattern formation (in still compartments). The distinct feature of the BZ reaction is that it possible to observe development of complex patterns in time and space by naked eye on a very convenient human time scale of dozens of seconds and space scale of several millimetres. The BZ reaction can generate up to several thousand oscillatory cycles in a closed system, which permits studying chemical waves and patterns without constant replenishment of reactants. The sensitivity of the system to the external conditions changes make possible to investigate the change the pattern formation process in response on the modulating agent strongest factor (shape geometry in our case).
There are several hypothesis followed the target pattern propagation and wave evolution in the system [3,4], but most of them concentrate on the cascade of the chemical transformations [5,6] or process taking place in the reaction-diffusion system model [7].Our investigation create new factors to forecast the chemical system behaviour under changed environment conditions and their impact on the main structure formation of waves. The formation of structures presented as the combination of two processes which was detected by independent analysis of blue and red channel of the colour camera (Figure 1.). While blue and green colour cannel follows the Fe[(phen)3]2+ and Fe[(phen)3]3+ - reaction catalyst and redox-indicator - concentration oscillation, oscillation in red colour channel was followed completely different behaving process. We suggest that the process observed in the red channel has the character of phase change and propose it to be connected with formation of Br2 taking place during all reaction evaluation time. Our hypothesis have an agreement with other scientists work [8,9]. In this work are present the experimental results to prove that BZ system wave structure evolution response on changes in geometrical conditions are not only a reflection of chemical kinetics modification, but also directly dependent on the phase transition derived due to structural changes in Br-contained reaction compounds. We propose a model of the process which explains most of the distinct aspects of the structure formation based on deduced hypothesis.
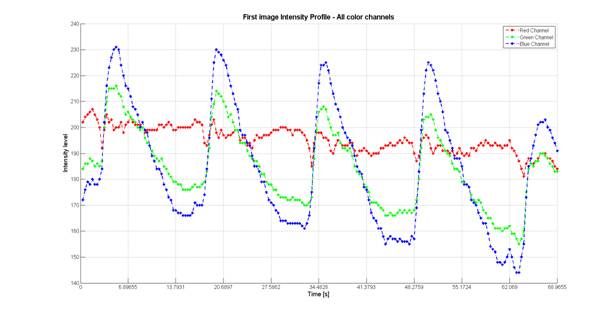
Figure 1. The wave evaluation course tracing in the tree colour channels demonstrate the reliable differences in the red colour channel. This fact allows us to suppose that along with changes in a chemical compound (have been traced by pixel intensity time oscillation in wave shape in green and blue colour channel) examined system is leading by some other accompanied process (phase transitions).
1. Dr. Jack Cohen Belousov-Zhabotinski Reaction Do-it-Yourself Kit, available at http://drjackcohen.com/BZ01.htm
2. B. Belousov, Biophysics, 9 (1959), pp. 306 – 311.
3. John J. Tyson, Springer-Verlag. New York: 1976.
4. Cross M. C., Reviews of Modern Physics, Vol. 65, No 3, July 1993, pp. 851-1130.
5. Richard J. Field and Richard M. Noyes, Journal of the American Chemical Society, 96:7:2001-2006, 1974.
6. Rovinsky A., Zhabotinsky A., Phys. Chem. 88(1984): 6081-6084.
7. H. Kitahata and K. Yoshikawa, J. Phys.: Condens. Mat., 17, S4239 - S4248 (2005): Chapter 6.
8. Vladimir K. Vanag, Anatol M. Zhabotinsky, and Irving R. Epstein, J. Phys. Chem. A 2000, 104, 11566-11577.
9. Tian Ma and Shouhong Wang, Mathematical Methods in Applied Sciences, 34:11(2011), 1381-1397.
This work was partly supported by the Ministry of Education, Youth and Sports of the Czech Republic – projects CENAKVA (No. CZ.1.05/2.1.00/01.0024) and CENAKVA II (No. LO1205 under the NPU I program), by Postdok JU CZ.1.07/2.3.00/30.0006 and GAJU Grant (134/2013/Z 2014 FUUP).