Structure of LLT1, a ligand
for human NKR-P1, and its variability under various conditions
Tereza Skálová1*, Jan Bláha2,
Karl Harlos3, Jarmila Dušková1,
Tomáš Kovaľ4, Jan Stránský1, Jindřich Hašek1, Ondřej
Vaněk2 and Jan Dohnálek1,4
1Institute of Biotechnology, Academy
of Sciences of the Czech Republic, v.v.i., Vídeňská
1083, 142 20 Praha 4, Czech Republic,
2Department of Biochemistry, Faculty of Science, Charles University
Prague, Hlavova 8, 128 40 Praha
2, Czech Republic,
3Division of Structural Biology, The Wellcome
Trust Centre for Human Genetics, University of Oxford, Roosevelt Drive, Oxford
OX3 7BN, United Kingdom
4Institute of Macromolecular Chemistry, Academy of Sciences of the
Czech Republic, v.v.i., Heyrovského
nám. 2, 162 06 Praha 6, Czech
Republic,
t.skalova@gmail.com
Natural killer cells (NK cells) are large granular lymphocytes – a type of white blood cells. They are able to kill virally infected, stressed or tumor cells. Unlike T-cells, the activity of NK cells is innate, they do not need to have previous experience with a tumor – they are natural killers.
NKR-P1 (CD161) is a receptor on a surface of human NK cells. LLT1 is a ligand for NKR-P1 receptor, expressed primarily on activated lymphocytes and antigen presenting cells. The interaction of the ligand with the receptor inhibits NK cell cytotoxicity; however, it may have also activation effects in some cases. Extracellular domains of both binding partners, NKR-P1 and LLT1, have C-type lectin like (CTL) fold.
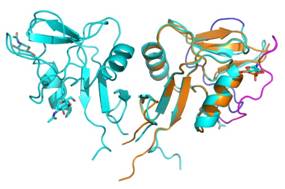
Using X-ray diffraction, we determined four structures of LLT1 [1] from protein produced in HEK293S GnTI- cells. The protein with GlcNAc2Man5 glycosylation packs into hexamers (consisting of three dimers) in crystals. The protein deglycosylated after the first N-acetylglucosamine was found in our crystal structures in forms of dimers (in pH 7.0) and monomers (in pH 3.5).
The LLT1 structures (Figure 1) show that LLT1 follows the “classical” mode of dimerization known from other structures with the same fold (CD69 [2], Clr-g [3]). The series of the LLT1 structures bring insight into variability of the dimerization interface, flexibility of the outer long loop of the CTL domain and influence of glycosylation on the structure.
This study was supported by BIOCEV CZ.1.05/1.1.00/02.0109 from the ERDF, by the Czech Science Foundation (project 15-15181S), by the Ministry of Education, Youth and Sports of the Czech Republic (grant LG14009), by Charles University (UNCE 204025/2012, SVV 260079/2014), High Education Development Fund (FRVS 669/2013), BioStruct-X (EC FP7 project 283570) and Instruct, part of the European Strategy Forum on Research Infrastructures (ESFRI) supported by national member subscriptions.
1. Skalova et al., Acta Crystallographica D, in press.
2. Natarajan et al., Biochemistry, 2000, 39, 14779-14786.
3. Skalova et al., J. Immunology, 2012, 189, 4881-4889.