Carborane-Based Inhibitors of Carbonic Anhydrases
Jiří Brynda1,2, Petr
Pachl1,2, Václav Šícha3,
Milan Fábry1, Bohumír Grüner3, Petr Cígler2, Pavlína Řezáčová1,2
1Institute of Molecular Genetics Academy of Sciences of the Czech Republic, v.v.i.
2Institute of Organic Chemistry and Biochemistry AS CR, v.v.i.
3Institute of Inorganic Chemistry, Academy of Sciences of the Czech Republic, v.v.i.
Carbonic anhydrases are ubiquitous zinc metalloenzymes that catalyse interconversion between carbon dioxide and the bicarbonate ion. So far, 15 human carbonic anhydrase (CA) isoforms have been identified displaying significant differences in catalytic activity, subcellular localization and tissues expression. They play key roles in intracellular and extracellular pH homeostasis, in the transport of CO2 and bicarbonate in respiration, and in several biochemical pathways where either CO2 or bicarbonate is required. Immense experimental evidence also suggests a role of CAs in various pathological processes and many CA isoenzymes have thus become established diagnostic and therapeutic targets.
The traditional CA inhibitors contain a sulfonamide or sulfamide moiety that coordinates Zn2+ cation located in the CA active site. Most of the currently used CA inhibitors lack selectivity, and their use has some problematic side effects. This opens new round of design of inhibitors that can block specific isozymes. Although the conical active-site clefts of different human CA isoezymes are highly homologous, variations exists in the amino acid composition at the entrance to the active site. As a result of their differing in shape and hydrophobicity, these surface pockets can be exploited to design specific inhibitors.
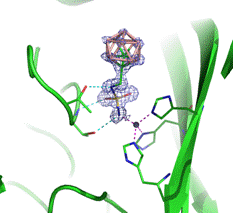

In conclusion, our results suggest that carborane-based compounds are promising lead structures for the development of inhibitors of CA isozymes. Our experiments demonstrated that various types of hydrophobic, space-filling carborane clusters can be accommodated in the CA active site and that substitution with an appropriately attached sulfamide group and other substituents leads to compounds with high selectivity for the cancer-specific CAIX isozyme over the widespread CAII isozyme. Crystal structures confirmed our hypothesis that three-dimensional scaffolds could be efficiently used in CA inhibitors and provided structural information that can be applied to the structure-based design of specific CAIX inhibitors.
The study is supported by the Czech Science Foundation (GA CR) grant no. 15-05677S.