Influence of the substitution effects in the pyridine ring on the reactivity of the trans-[Pt(NH3)2(pyr)Cl]+ complex
Olga Dvořáčková,1,2 and Zdeněk
Chval1
1Faculty of Health and Social Studies, University of South Bohemia, J. Boreckého 27, 370 11 České Budějovice, Czech Republic,
2Institute of Physics and Biophysics, Faculty of Science, University of South Bohemia, Branišovská 31, 370 05 České Budějovice, Czech Republic
Platinum anticancer complexes are administered in its inactive form and the hydrolysis step is needed for their activation. The activated drug reacts rapidly with DNA or proteins and the hydrolysis step is the rate determining step of the whole process. Thus, the modification of the speed of hydrolysis is one of the ways of a new drug development.
In the present work we have studied the kinetics of the aquation reaction on the trans-[Pt(NH3)2(pyr-X)Cl]+ complex (X = OH‑, Cl‑, NO2‑, NH2, SH, CH3). All possible positions ortho-, meta- and para- of the substituent X in the pyridine ring were considered and reaction energy profiles, atomic charges, electron densities at bond critical points, ligand binding energies were calculated. All the structures along the reaction pathways were fully optimized using B3LYP/MWB-60(f)/6-31+G* method. Single point energies and molecular properties were evaluated by B3LYP/MWB-60(2fg)/6-311++G(2df,2pd) method.
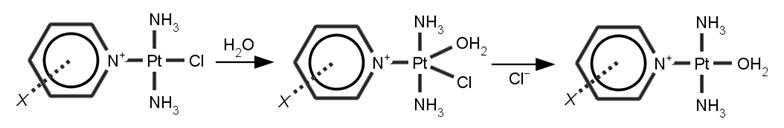
Figure 1: Reaction mechanism
of the aquation
reactions.
The substituent ligand influences electron density on the pyridine ring and thus the electron donating ability of the heterocyclic nitrogen. The substituent ligand on the pyridine ring can be ordered according to their ability to promote the aquation reaction. The largest selectivity offers the ortho- position which offers almost 400 times difference in the rate of the reaction between the fastest o-NH2 and the slowest o-Cl‑ ligand: NH2 > OH‑ > SH‑ > CH3 > NO2‑ > H > NO2‑~ Cl.
This work is supported by the Czech Science Foundation (grant No. 208/12/0622).