Structural
properties of NK receptors and ligands with C-type lectine-like
fold
T. Skálová1,
P. Kolenko1, J. Dušková1, T. Kovaľ1, J. Hašek1,
J. Stránský1,2, J. Dohnálek1
1Institute
of Macromolecular Chemistry,
v.v.i., 16206
Praha 6, Czech Republic
2Faculty
of Nuclear Sciences and Physical Engineering,
t.skalova@gmail.com
Natural
killer cells (NK cells) are blood corpuscles, more precisely, a sort of
lymphocytes. They comprise 5-10% of lymphocytes in blood and their role in the
immune system is to discover and kill cancer cells and cells infected by
viruses.
This work
is aimed at a class of NK receptors, i.e. receptors on the surface of NK cells,
which have a special fold: C-type lectin-like fold
(CTL fold, (1)). More generally, other receptors, not only NK receptors, but
with CTL fold, will be mentioned included in the presented analysis. The role
of NK receptors with CTL fold is to mediate contact with other cells, in order
to kill infected or cancer cells. NK receptors with CTL fold interact with protein
ligands, which are of the same, CTL, fold, and are located on surface of
partner cells.
During
recent years, we have solved structures of several receptors/ligands with CTL
fold (high resolution structure of human CD69 (2), mouse NKR-P1A (3, 4), mouse Clr-g (5)), and other structures are in progress. These
structures inspire us to study 1) CTL fold, its characteristics and its
variability, 2) Types of oligomerization of CTL
receptors and ligands (Figure 1), and 3) Rules of formation of CTL
receptor-ligand complexes.
It was
found that the dimerization mode of CTL proteins is
very variable, while complexation of structurally
known CTL protein-protein complexes happens in the same area of monomers, in
the part distant to N and C terminal region.
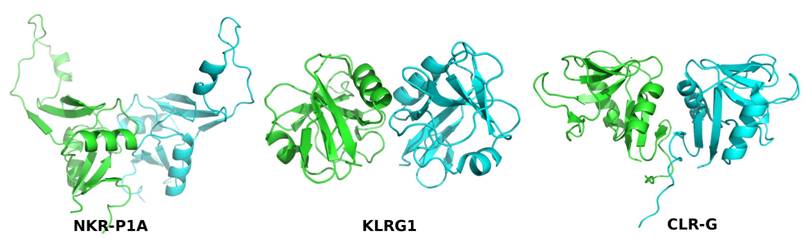
Figure 1. Comparison of dimerization modes of three
mouse CTL proteins: NK receptor NKR-P1A (PDB code 3M9Z), NK receptor Klrg1
(3FF9), and a ligand for NK receptor NKR-P1F: Clr-g (3RS1).
This work was supported by the Czech Science Foundation (P302/11/0855), and the Ministry of Education, Youth and Sports of the Czech Republic (CZ.1.07/2.3.00/30.0029).
1. A. N. Zelensky and J. E.
Gready, The C-type lectin-like domain superfamily, FEBS Journal, 272, (2005), 6179–6217.
2. P. Kolenko
et al., The high-resolution structure of the extracellular domain of human CD69
using a novel polymer, Acta Crystallogr., F65, (2009), 1258-1260.
3. P. Kolenko et al., Molecular
architecture of mouse activating NKR-P1 receptors, J. Struct. Biol., 175(3), (2011), 434-441.
4. P. Kolenko et al., Structure of the H107R variant of the extracellular domain
of mouse NKR-P1A at 2.3 Å resolution, Acta
Crystallogr., F67, (2011),
1519-1523.
5. T. Skalova et al., Mouse Clr-g, a Ligand for NK Cell Activation Receptor NKR-P1F: Crystal Structure and Biophysical Properties, J. of Immunology, 189(10), 2012, 4881-4889.