Computational
Study of Interactions of Organic Matter and Biomolecules with Mineral Surfaces
H. Barvíková1, M. Předota1, O. Kroutil1,2, Z. Chval2 and Babak Minofar1,3
1Faculty of Science, University of South Bohemia, Branišovská 31, 370 05 České Budějovice, Czech Republic
2Faculty of Health and Social
Studies, University of South Bohemia, J. Boreckého
27, 37 011 České Budějovice,
Czech Republic
3Institute of Nanobiology and Structural Biology of GCRC ASCR,v.v.i., Zámek
136, 373 33 Nové Hrady.
* email: h.barvikova@centrum.cz
Humic acids and humates are characterized by heterogeneous and complex structures with different functional groups including acidic, hydrophilic and hydrophobic groups. Humic acids interact with both organic and inorganic substances such as nutrients, metals, hydrophobic organic compounds and mineral surfaces. Understanding their structure and interactions can provide important information about their degradability, toxicity and transport properties. These substances are also known as one of the major causes of so-called ‘bio-fouling’ of nanofiltration and reverse osmosis membranes used for water purification, desalination and wastewater treatment.
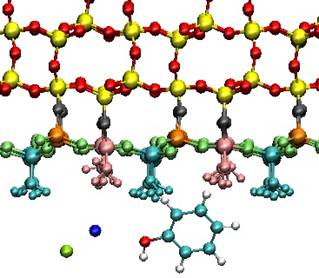
We carried out molecular dynamics simulations of interactions of quartz surfaces with aqueous solutions of ions and small organic molecules representing basic building blocks of larger biomolecules and functional groups of organic matter. As model molecules, benzoic acid, phenol, and salicylic acid were chosen.
Focusing our attention on leading interactions and roles of participating atoms and groups identified in the complexes, we studied interactions of molecules with surfaces for a set of surface charge densities corresponding to the experimentally or environmentally relevant ranges of pH values employing molecular mechanics, molecular dynamics and ab initio techniques. Simulated quartz surfaces covered the range of surface charge densities 0.00, ‑0.03, -0.06 and -0.12 C/m2, approximately corresponding to pH values 4.5, 7.5, 9.5 and 11.
We found increasing water structuring within two water layers closest to the surface as well as sodium ions adsorption, both depending on increasing surface charge density. The adsorption of organic molecules is rather week, but specific binding patterns were identified.
Our future objective is modeling interactions of nucleic acid building blocks, polycyclic aromatic hydrocarbons, organic matter and mixtures of solvents with quartz and rutile surfaces applied on environmentally and technologically important systems.
We gratefully acknowledge support from the Grant Agency of the Czech Republic 13-08651S and P208/12/0622.