Reaction mechanism principles of the bifunctional plant nuclease TBN1
T. Koval1, P. Lipovova3,
T. Podzimek3,4, J. Matousek4, J.
Duskova2, T. Skalova2, J. Hasek2 and J.
Dohnalek1,2
1Institute
of Physics, Academy of Sciences of the Czech Republic,v.v.i., Na Slovance 2,182
21 Praha 6,
2Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic,v.v.i., Heyrovskeho nam. 2, 162 06 Praha 6, Czech Republic
3Institute of Chemical Technology, Technicka 5, 166 28 Praha 6, Czech Republic
4Institute of Plant Molecular Biology, Biology Centre, Academy of Sciences of the Czech Republic,v.v.i., Branisovska 31, 370 05 Ceske Budejovice, Czech Republic
koval.tomas@gmail.com
Bifunctional nuclease TBN1 (UniProt sequence accession no. Q0KFV0) from Solanum lycopersicum (tomato) is a Zn2+- dependent plant glycoprotein composed of 277 amino acids with a molecular mass of 31.6 kDa (about 37 kDa when glycosylated). TBN1 belongs to plant nuclease I family and plays an important role in specific apoptotic functions, vascular system development, stress response and tissue differentiation in plants [1]. In addition, TBN1 exhibits anticancerogenic properties [2]. Therefore, a detailed structural study of this enzyme can contribute to development of new drugs for cancer, bacterial and viral disease treatment. Nuclease P1 from Penicillium citrinum with 24% sequence identity, the structure of which is known (PDB ID 1ak0) [3], is probably the closest structural homologue of TBN1.
Heterologous expression of TBN1 in tobacco leaves yields amounts and quality of the enzyme suitable for structural studies. Crystals with sufficient quality for X-ray diffraction analysis can be obtained. The first diffraction experiments were performed using an in house Gemini Enhanced Ultra diffractometer with the Atlas CCD detector (Oxford Diffraction) and three different crystal morphologies were identified (orthorhombic, rhombohedral and trigonal). Datasets for structural analysis were collected at the synchrotron radiation source BESSY II (Helmholtz-Zentrum Berlin), beam line MX-14-1, with a MARmosaic CCD detector. Presence of zinc in the protein was confirmed by X-ray fluorescence and an absorption edge scan and two MAD datasets (for a rhombohedral and a trigonal crystal) were collected. The phase problem was solved using the SHELXC, D and E program suite [4]. A cluster of zinc ions was identified and a preliminary structure model was built by placing the P1 nuclease structure into the experimental electron density using MOLREP [9]. Building and refinement of this structure was limited [6,7]. The trigonal crystal diffracted to 2.15 Å resolution and the final model was built and refined using this data. The TBN1 structure is mainly α-helical with three Zn ions placed in the active site at the bottom of the wide groove. Three oligosaccharides bonded on the surface serve primarily as a shielding of the hydrophobic regions and therefore contribute to solubility of the enzyme. The main differences between TBN1 and P1 are in the composition of the side chains around the active site, in the glycosylation pattern and also in the shape and in the electrostatic potential on the surface.
TBN1 acts as phosphodiesterase
cleaving the bond between phosphorus and
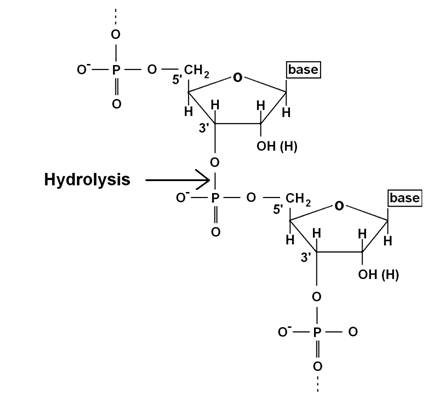
Figure 1 TBN1 cleaves the bond
between the 3’ hydroxyl group and phosphate in nucleic acids by hydrolysis.
The work on this project was
supported by the Czech Science Foundation, project no. 310/09/1407. We also
thank the Grant Agency of the Czech Republic, project no. 202/06/0757 and
project no.521/09/1214 and by the EC under ELISA grant agreement number 226716
(synchrotron access, projects 09.2.90262 and 10.1.91347). We also gratefully acknowledge support from Praemium Academiae of AS CR,
Institution research plan AVOZ10100521 of the Institute of Physics, Institution
research plan AV0Z50510513 of the Institute of Plant Molecular Biology, Biology
Centre. The authors wish to thank Dr. U. Müller of
the Helmholtz-Zentrum
1. J. Matousek, P. Kozlova, L. Orctova, A. Schmitz, K. Pesina, O. Bannach, N. Diermann, G. Steger,
D. Riesner, Biol. Chem., 388, (2007), 1–13.
2. J. Matousek, T. Podzimek, P. Pouckova, J. Stehlik, J. Skvor, P. Lipovova, J. Matousek, Neoplasma, 57,
(2010), 339-348.
3. C. Romier, R. Dominguez, A. Lahm, O. Dahl, D. Suck, Proteins, 32, (1998), 414–424.
4. G.
M. Sheldrick, Acta Cryst.,
A64, (2008), 112-122
6. T. Koval, P. Lipovova, T. Podzimek, J. Matousek, J. Duskova, T. Skalova, A. Stepankova, J. Hasek, J.
Dohnalek, Acta. Cryst. F67, (2011), 124-128.
7. J. Dohnalek, T. Koval, P. Lipovova, T. Podzimek, J. Matousek, J. Synch. Rad., 18, (2011), 29-30.