THERMODYNAMICAL
ANALYSIS OF THE MUTANT CV-IIL LECTINS FROM CHROMOBACTERIUM
VIOLACEUM
Eva Dejmková 1, Martina Pokorná 1,
Michaela Wimmerová1,2
1National
Centre for Biomolecular Research and 2Department of Biochemistry,
Lectins are proteins or glycoproteins of non-immune
origin capable agglutinate cells. They are able to specifically recognize saccharides
with high affinity. However, the major function of lectins appears to be in the
cell recognition process, they generally recognize diverse sugar structures and
mediate a variety of biological processes. Bacterial lectins play a crucial
role in recognition of sugar moieties on the host cell surface and consequently
can cause bacterial adhesion. An attractive approach is the use of agents that interfere with the
ability of the bacteria to adhere to the host cell surface, since such adhesion
is one of the initial stages of the infectious process. However, the process of ligand binding is very complex and
complicated, deeper understanding of the thermodynamics of lectin-saccharide
interaction can be useful for the molecular design of potent anti-adhesion
therapeutics.
 Chromobacterium violaceum
is an opportunistic pathogen that commonly occurs in water and soil in tropical
and subtropical regions. Infection caused by this bacterium can be fatal for
immunocompromised people and children. Bacterial unusual resistance to antibiotics
is the reason of high mortality.
Chromobacterium violaceum
is an opportunistic pathogen that commonly occurs in water and soil in tropical
and subtropical regions. Infection caused by this bacterium can be fatal for
immunocompromised people and children. Bacterial unusual resistance to antibiotics
is the reason of high mortality.
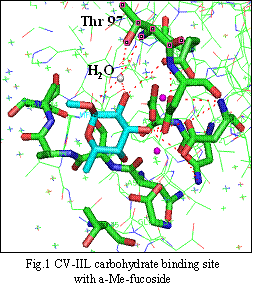
The lectin from C. violaceum named CV-IIL is a tetramer (the structural functional unit is a dimer), each monomer is composed of 113 amino acids. This lectin shows ability to bind L-fucose (6-deoxy-L-galactose) and D-mannose with high affinity. Each monomer contains two calcium ions in the carbohydrate binding site, which mediate binding of the saccharides. The crystal structure of CV-IIL demonstrates that there is also one water molecule, which plays a special role by bridging sugar with backbone nitrogen atom and also with side chain of the amino acid threonine in position 97. (Fig.1) [1] Therefore, several representatives from different groups of amino acids were chosen for the mutagenesis in this position 97.
The complete thermodynamical analysis was
performed using isothermal titration microcalorimetry method (ITC 200,
Microcal). From the single measurement we can obtain and consequently calculate
important thermodynamical values (enthalpy, entropy, Gibbs free energy) as well
as binding affinity and stoichiometry of the lectin-saccharide interaction. The
interaction with high affinity ligand α-Me-fucoside was measured in case
of each prepared mutant lectin at different temperatures in the range from 10
to
∆S° = ∆S solv + ∆S config + ∆S rot + ∆S transl [2]
The two
major contributions to the binding entropy are the change in conformational and
the solvation entropy, which will be discussed. However, the values of the
Gibbs free energy are very similar, the difference in enthalpy and entropy
contributions were marked in some cases.
The work is supported by Ministry of
Education (MSM0021622413, ME08008) and Czech Science Foundation (GA/303/09/1168)
of the
1. Pokorná M., Cioci G., Perret S, Rebuffet E., Kostlánová N., Adam J., Gilboa-Garber N., Mitchell E.P., Imberty A., Wimmerová M., Unusual Entropy-Driven Affinity of Chromobacterium violaceum Lectin CV-IIL toward Fucose and Mannose, Biochemistry 45 (24) 7501 - 7510, 2006
2. Chervenak M.C., Toone E.J., Calorimetric Analysis of the Binding of Lectin with Overlapping Carbohydrate-Binding Ligand Specifities, Biochemistry 34 5685 - 5695, 1995