Reduction mechanism of the Pt(IV) Satraplatin
derivates by guanosine monophosphate; quantum mechanical study
Filip Šebesta, Jaroslav V. Burda
Faculty of Mathematics and Physics, Charles University, Ke Karlovu 3, 121 16 Prague 2, Czech Republic
The platinum IV compound Satraplatin
(JM216) was selected for clinical development because of: a) high cytotoxic
activity in vitro against several solid tumor cell lines, including cisplatin
resistant ones; b) in vivo oral antitumor activity against a variety tumor
models; c) a relatively mild toxicity profile and oral bioavailability. In
Phase 2 clinical trials, satraplatin showed activity against several different
cancers, including prostate, ovarian, and small cell lung cancers [1].
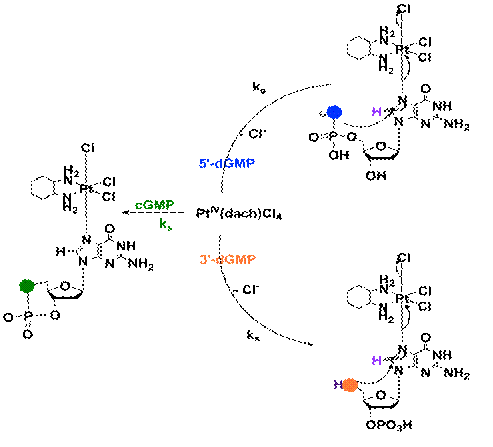
When
PtIV(dach)Cl4 reacts with dGMP or GMP the pH drops form 8.3 to 3.4,
and yellow crystals are formed. The crystals were identified as PtII(dach)Cl2
by IR analysis (3267, 3190, 3066, 2936, 2863, and 1564 cm-1). This indicates
that both dGMP and GMP can reduced Pt(IV) to Pt(II) [2].
Quantum
chemical tool was used to confirm the suggested reactions and explore the
detailed reduction mechanism. Both thermodynamic and kinetic characteristics
were determined at several computational levels. The AIM,
NBO and frequency analyses were used for examination of all the individual
complexes.
(1) Choi, S.; Filotto, C.; Bisanzo, M.; Delaney, S.; Lagasee, D.; Whitworth, J. L.; Jusko, A.; Li, C.; Wood, N. A.; Willingham, J.; Schwenker, A.; Spaulding, K. Inorg. Chem. 1998, 37, 2500-2504.
(2) Angelov, D.; Spassky, A.; Berger, M.;
Cadet, J. J. Am. Chem. Soc. 1997, 119, 11373-11380.