HOW COULD FLEXIBILITY OF RNA THREE WAY
JUNCTION FROM THE GTP-ASE ASSOCIATED CENTER OF 50S ASSIST TO THE ACCOMMODATION
OF THE TRNA INTO THE RIBOSOME?
I. Beššeová1,2, K. Réblová1 and J. Šponer1
1 Institute of Biophysics, Academy of Sciences of
the Czech Republic, Kralovopolska 135, 61265 Brno
2 Gilead Sciences&IOCB Research Center, Institute of Organic
Chemistry and Biochemistry, Academy of Sciences of the Czech Republic,
Flemingovo namesti 2, 166 10, Prague 6
We studied RNA three-way junction
The aim of the study was to analyze the intrinsic flexibility of the H42-H44 rRNA and to consider it in the ribosomal context in available crystal structures of the ribosome including tRNAs, elongation factors, etc. The flexibility of the RNA is evaluated based on preferred stochastic thermal fluctuations sampled in unrestrained simulations. The simulations thus identify the intrinsic low-energy deformation modes of the molecules that can co-operate with the surrounding ribosomal elements to achieve the functional dynamics.
MD reveals that GAC 3WJ possesses significant anisotropic hinge-like flexibility [1]. Projecting observed movement into the ribosome, H43/H44 is flexible in direction towards and away (closing-opening geometry path of the GAC 3WJ) of the large ribosomal subunit, see the Figure. When the H42-H44 domain is in the overall "closed” conformation, the tip of the hairpin loop of H89 can fit into the groove defined by the docking of the hairpin loops of H43/44. However, such contact is only seen in the 2AW4 crystal structure of vacant E.coli ribosome [2]. In other crystal structures the distance between the H89 and GAC RNA varies widely (it even exceeds in some cases 10 Å) [3, 4]. The experimental structures show a wide range of positions sampling a set of more inward and more outward structures with respect to the A-site of the large subunit and H89. The range of observed positions coincides well with the anisotropic flexibility direction predicted by MD.
The simulations show that flexibility of the GAC RNA stems from the 3WJ and includes also the H42 stem region below the 3WJ and above the conserved H42-H97 tertiary interaction. The GAC rRNA could undergo large-scale rapid thermal fluctuations or structural adaptations to facilitate gliding of the tRNA to H89, which lead it into its functional destinations (A/A state) [5], see the Figure.
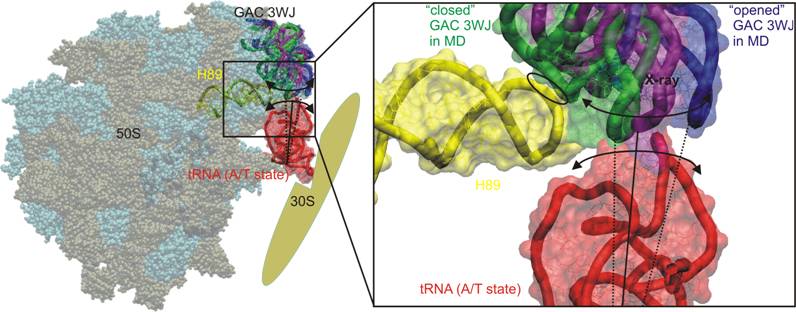
Figure 1: Left - Large ribosomal subunit (RNA in tan, proteins in cyan, pdb code 2WRO [2]) from Thermus termophilus including tRNA in the A/T state (in red, 2WRN [2]). The GAC 3WJ in the X-ray data is in purple, opened and closed geometry of the junction occurred in the MD simulations is shown in green and blue, respectively. Right - Detailed view on coordinated hypothetical movement of the tRNA and GAC 3WJ. The closed MD geometry of the GAC 3WJ forms contact with H89 (marked by ellipse), which might be a key point, where GAC 3WJ deliver tRNA to H89 (in yellow).
[1] I. Besseova, K. Reblova, N. B. Leontis & J. Sponer, Nucleic Acids Res., 38 (2010) 6247-6264.
[2] B. S. Schuwirth, M. A. Borovinskaya, C. W. Hau, W. Zhang, A. Villa-Sanjurjo, J. M. Molton & J. H. D. Cate, Science, 310 (2005) 827-834.
[3] T. M. Schmeing, R. M. Voorhees, A. C. Kelley, Y.
G. Gao, F. V. Murphy, J. R. Weir & V. Ramakrishnan, Science, 326 (2009) 688-694.
[4] D. J. Klein, P. B. Moore & T. A. Steitz, J.Mol.Biol., 340 (2004) 141-177.
[5] K. Y. Sanbonmatsu, S. Joseph & C. S. Tung, Proc. Natl. Acad. Sci. U. S. A., 102 (2005) 15854-15859.