Supramolecular Assemblies Formed by Diolein and Stearyl Alcohol
V. Andrushchenko1, W. Pohle2, D.R. Gauger2 and P. Bouĝ1
1Institute of Organic Chemistry and Biochemistry, Academy of Sciences,
Flemingovo nam. 2, Prague, 16610, Czech Republic
2 Institute of Biochemistry & Biophysics, Friedrich-Schiller University Jena, Philosophenweg 12, D-07743 Jena, Germany
andrushchenko@uochb.cas.cz
Diolein (1,2-dioleoylglycerol, DOG) and stearyl alcohol (1-octadecanol, ODA) are typical amphiphiles, which can be conveniently used as glycolipid models. They have some structural similarities, namely both contain 18 carbon atoms in the chain and have a single hydroxyl group. Despite these similarities, they assemble into very different supramolecular structures. It was shown by a combination of IR spectroscopy and MD/QM simulations that the assemblies formed by DOG are characterized by low structural order and very weak hydrogen bonding, while those formed by ODA, on the contrary, exhibit highly ordered structures stabilized by strong hydrogen bonds (Figure 1).
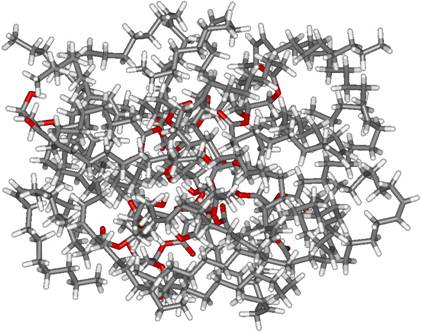
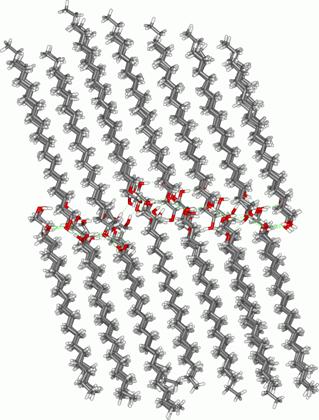
Figure 1. Resulting MD
structures of DOG (left) and ODA (right) assemblies.
Support from the Grant Agency of the Czech Republic (grant P208/10/0559 (VA), P208/11/0105 (PB)) and Academy of Sciences (grant M200550902 (PB)) is gratefully acknowledged.