Human carbonic anhydrase complexes with isoquinoline inhibitors
P. Mader1,
J. Brynda1, R. Gitto2, A. Chimirri2
1Institute
of Molecular Genetics AS CR,
2Dept. Farmaco-Chimico, Università
di Messina, Messina,
mader@img.cas.cz
Keywords: carbonic anhydrase inhibitors, isoquinolines,
Abstract
Human carbonic anhydrases (CAs) form a family of 14 zinc-containing enzymes that catalyze rapid conversion between carbon dioxide and bicarbonate. This reaction plays role in many physiological processes, such as respiration, regulation of pH and many other biological processes requiring carbon dioxide or bicarbonate. Human isozyme CA II is a cytosolic enzyme and belongs to the most studied isoforms. It is traditionally purified from red blood cells but it has a wide tissue distribution and is found in various organs and cell types [1]. CA II deficiency is associated with osteoporosis, renal tubular acidosis, and cerebral calcification. The transmembrane isoform CA IX has been shown to be linked with carcinogenesis. CA IX is physiologically expressed in the epithelia of the gastrointestinal tract, however it is expressed ectopically in carcinomas derived from kidney, lung, cervix, uteri, oesophagus, breast, and colon. This isoform has been shown to be strongly over-expressed in hypoxic tumors, where it participates in tumor cell environment acidosis and contributes to malignant progression and poor treatment outcome. Designing isoform-selective inhibitors could thus provide potent therapeutics namely for treatment of cancer. Progress in structural studies [2] of these two physiologically and pathophysiologically important isoenzymes in complex with selective isoquinoline inhibitors will be discussed. Elucidation of inhibitor binding to various isoforms can help in rational drug design of carbonic anhydrase inhibitors.
References
1. Sly and Hu Annu. Rev. Biochem. 64 (1995), pp. 375–401
2. Gitto R., Agnello S., Ferro S., Luca L. De, Vullo D., Brynda J., Mader P, Supuran C.T., Chimirri A., J .Med. Chem., in press
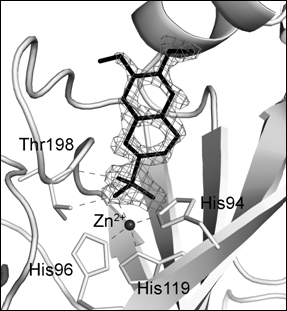
Figure
1. Isoquinoline-sulfonamide derivative binding to
active site of human CAII